"Materials": Difference between revisions
Shizuka Anan (talk | contribs) No edit summary |
Shizuka Anan (talk | contribs) No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<div style="padding: 10px; width: 730px; color: #3ca83c; background-color: | <div style="padding: 10px; width: 730px; color: #3ca83c; background-color:#3ca83c"> | ||
#3ca83c"> | |||
[[Image:MARIMOD3ca83c.png| 150px]] | [[Image:MARIMOD3ca83c.png| 150px]] | ||
[[Biomod/2013/Hokkaido | <font face="trebuchet ms" style="color:#FFFFFF" | | ||
| |||
size="4"> '''Home''' </font>]] | [[Biomod/2013/Hokkaido | <font face="trebuchet ms" style="color:#FFFFFF" size="4"> '''Home''' </font>]] | ||
[["What's "Marimo"?" | <font face="trebuchet ms" style="color:#FFFFFF" | [["What's "Marimo"?" | <font face="trebuchet ms" style="color:#FFFFFF" size="4"> '''What's Marimo''' </font>]] | ||
size="4"> '''What's Marimo''' </font>]] | |||
| | ||
[["Design" | <font face="trebuchet ms" style="color:#FFFFFF" size="4"> | [["Design" | <font face="trebuchet ms" style="color:#FFFFFF" size="4">'''Design''' </font>]] | ||
'''Design''' </font>]] | |||
<br> | <br> | ||
| |||
| |||
| | ||
[["Materials" | <font face="trebuchet ms" style="color:#FFFFFF" | | ||
[["Materials" | <font face="trebuchet ms" style="color:#FFFFFF" size="4"> '''Materials''' </font>]] | |||
size="4"> '''Materials''' </font>]] | |||
| | ||
[[" | [["People" | <font face="trebuchet ms" style="color:#FFFFFF" size="4">'''People''' </font>]] | ||
''' | |||
| | ||
[["Sponsors" | <font face="trebuchet ms" style="color:#FFFFFF" size="4">'''Sponsors''' </font>]] | |||
[["Sponsors" | <font face="trebuchet ms" style="color:#FFFFFF" size="4"> | |||
'''Sponsors''' </font>]] | |||
</div> | </div> | ||
---- | |||
===[[Image:大見出し.png| 30px]] <font size="5">Preparation of Ring-Shaped Microtubule Assemblies</font>=== | |||
====[[Image:小見出し.png|18px]] <font size="4">Purification of tubulin</font>==== | ====[[Image:小見出し.png|18px]] <font size="4">Purification of tubulin</font>==== | ||
<p> | <p> | ||
Tubulin was purified from porcine brain through two cycles of polymerization- | Tubulin was purified from porcine brain through two cycles of polymerization-depolymerization <br> | ||
using a high-molarity buffer (1 M PIPES, 20 mM EGTA, 10 mM MgCl<sub>2</sub>; pH adjusted to 6.8); <br> | |||
depolymerization <br> | |||
using a high-molarity buffer (1 M PIPES, 20 mM EGTA, 10 mM MgCl<sub>2</sub>; pH | |||
adjusted to 6.8); <br> | |||
high-molarity PIPES buffer (HMPB). | high-molarity PIPES buffer (HMPB). | ||
</p> | </p> | ||
| Line 52: | Line 33: | ||
<font size="3">1.Preparation of Brain homogenate</font> | <font size="3">1.Preparation of Brain homogenate</font> | ||
---- | ---- | ||
Porcine brains were purchased from a local slaughterhouse, and conserved | Porcine brains were purchased from a local slaughterhouse, and conserved before<br> | ||
before<br> | |||
use in ice-cold PBS (20 mM Na-phosphate, 150 mM NaCl, pH7.2). Brains were<br> | use in ice-cold PBS (20 mM Na-phosphate, 150 mM NaCl, pH7.2). Brains were<br> | ||
cleaned of blood clots with , weighed, and transferred to a homogenizer | cleaned of blood clots with , weighed, and transferred to a homogenizer (nissei, AM-7).<br> | ||
Cold (4 °C) depolymerization buffer (DB) (50 mM 2-[N-morpholino] ethanesulfonic acid,<br> | |||
(nissei, AM-7).<br> | 1 mM CaCl<sub>2</sub>, pH 6.6) was added at a ratio of 1 mL/g of brain tissue. The mixture<br> | ||
Cold (4 °C) depolymerization buffer (DB) (50 mM 2-[N-morpholino] ethanesulfonic | |||
acid,<br> | |||
1 mM CaCl<sub>2</sub>, pH 6.6) was added at a ratio of 1 mL/g of brain tissue. | |||
The mixture<br> | |||
was homogenized eight times at 50000 rpm for 15 s.<br> | was homogenized eight times at 50000 rpm for 15 s.<br> | ||
</p> | </p> | ||
| Line 72: | Line 45: | ||
---- | ---- | ||
Brain homogenates were then centrifuged at 12,000 rpm for 60 min at 4 °C. <br> | Brain homogenates were then centrifuged at 12,000 rpm for 60 min at 4 °C. <br> | ||
The supernatants were pooled and supplemented with an equal volume of warm (37 | The supernatants were pooled and supplemented with an equal volume of warm (37 °C) <br> | ||
high-molarity PIPES buffer (HMPB) (1 M PIPES, 10 mM MgCl<sub>2</sub>, and 20 mM EGTA, pH 6.9) ,<br> | |||
°C) <br> | |||
high-molarity PIPES buffer (HMPB) (1 M PIPES, 10 mM MgCl<sub>2</sub>, and 20 mM | |||
EGTA, pH 6.9) ,<br> | |||
ATP (1.5 mM final), and GTP (0.5 mM final). ATP is included in this step to <br> | ATP (1.5 mM final), and GTP (0.5 mM final). ATP is included in this step to <br> | ||
remove motors and other proteins, which bind to microtubules in an ATP sensitive | remove motors and other proteins, which bind to microtubules in an ATP sensitive manner [2].<br> | ||
An equal volume (1/3 of the final volume) of pre-warmed to 37 °C anhydrous glycerol <br> | |||
manner [2].<br> | was added to this solution. This mixture was incubated in a 37 °C water bath for 1h. <br> | ||
An equal volume (1/3 of the final volume) of pre-warmed to 37 °C anhydrous | |||
glycerol <br> | |||
was added to this solution. This mixture was incubated in a 37 °C water bath | |||
for 1h. <br> | |||
The polymerized tubulin was then centrifuged at 44,000 rpm for 30 min at 37 °C. | The polymerized tubulin was then centrifuged at 44,000 rpm for 30 min at 37 °C. | ||
</p> | </p> | ||
| Line 95: | Line 58: | ||
---- | ---- | ||
The resulting microtubule pellets were resuspended in 100 mL of cold DB. <br> | The resulting microtubule pellets were resuspended in 100 mL of cold DB. <br> | ||
The depolymerized tubulin was subsequently centrifuged in at 34,000 rpm for 30 | The depolymerized tubulin was subsequently centrifuged in at 34,000 rpm for 30 min at 4 °C.<br> | ||
The supernatant from this centrifugation step was mixed with an equal volume <br> | |||
min at 4 °C.<br> | of the HMPB supplemented with ATP and GTP, followed by the addition of glycerol<br> | ||
The supernatant from this centrifugation step was mixed with an equal volume | (1/3 of final volume) as described above. The mixture was incubated in a 37 °C<br> | ||
water bath for 1h, and the polymerized microtubules centrifuged in at 44,000 rpm <br> | |||
<br> | for 30 min at 37 °C. Following the centrifugation, the microtubule pellets were resuspended <br> | ||
of the HMPB supplemented with ATP and GTP, followed by the addition of | in 15 mL of ice-cold BRB80 (80 mM PIPES, 1 mM MgCl<sub>2</sub>, 1 mM EGTA, pH 6.8) and then <br> | ||
incubated for a further 10 min on ice. After this polymerization step, the tubulin <br> | |||
glycerol<br> | was centrifuged in at 35,000 rpm for 30 min at 4 °C. The supernatant was collected <br> | ||
(1/3 of final volume) as described above. The mixture was incubated in a 37 | |||
°C<br> | |||
water bath for 1h, and the polymerized microtubules centrifuged in at 44,000 rpm | |||
<br> | |||
for 30 min at 37 °C. Following the centrifugation, the microtubule pellets were | |||
resuspended <br> | |||
in 15 mL of ice-cold BRB80 (80 mM PIPES, 1 mM MgCl<sub>2</sub>, 1 mM EGTA, pH | |||
6.8) and then <br> | |||
incubated for a further 10 min on ice. After this polymerization step, the | |||
tubulin <br> | |||
was centrifuged in at 35,000 rpm for 30 min at 4 °C. The supernatant was | |||
collected <br> | |||
and snap-frozen in 100 µL aliquots in liquid nitrogen. | and snap-frozen in 100 µL aliquots in liquid nitrogen. | ||
</p> | </p> | ||
<p> | <p> | ||
<font size="3">4.Determination of tubulin concentration</font> | |||
Tubulin concentration was determined by SDS-PAGE | ---- | ||
Tubulin concentration was determined by SDS-PAGE. | |||
</p> | </p> | ||
====[[Image:小見出し.png|18px]] <font size="4">Preparation of biotin | ====[[Image:小見出し.png|18px]] <font size="4">Preparation of biotin labeled tubulin</font>==== | ||
labeled tubulin</font>==== | |||
<p> | <p> | ||
1. Tubulin was thawed, and polymerized at 37 °C for 15 min.<br> | 1. Tubulin was thawed, and polymerized at 37 °C for 15 min.<br> | ||
2. Biotin-XX-SE (Molecular Probes, Cat. B-1606) was dissolved at | 2. Biotin-XX-SE (Molecular Probes, Cat. B-1606) was dissolved at 0.1 M in dry dimethyl sulfoxide (DMSO). <br> | ||
3. Biotin-XX-SE solution was added to tubulin, while pipetting to distribute it rapidly to a final concentration of 2 mM. incubate at 37 °C for 20 min.<br> | |||
0.1 M in dry dimethyl sulfoxide (DMSO). <br> | 4. Mixture was layered onto cushions and spun at 54,000 rpm for 1h at 37 °C.<br> | ||
3. Biotin-XX-SE solution was added to tubulin, while pipetting to | 5. Pellets were resuspended and spun cold, being careful to wash the cushion inter face well to remove all the biotin-XX-SE.<br> | ||
6. Tubulin was depolymerized and spun 40,000 rpm for 15 min at 4 °C.<br> | |||
distribute it rapidly to a final concentration of 2 mM. incubate at 37 °C for | 7. Steps (3) – (6) were performed to give once cycled biotin-tubulin.<br> | ||
8. Steps (3) – (6) were repeated to give twice cycled biotin-tubulin. The final pellet is resuspended in BRB80.<br> | |||
20 min.<br> | 9. The final biotin-tubulin is frozen and stored as per the cycled tubulin.<br> | ||
4. Mixture was layered onto cushions and spun at 54,000 rpm for 1h | |||
at 37 °C.<br> | |||
5. Pellets were resuspended and spun cold, being careful to wash | |||
the cushion inter face well to remove all the biotin-XX-SE.<br> | |||
6. Tubulin was depolymerized and spun 40,000 rpm for 15 min at 4 | |||
7. Steps (3) – (6) were performed to give once cycled biotin- | |||
tubulin.<br> | |||
8. Steps (3) – (6) were repeated to give twice cycled biotin- | |||
tubulin. The final pellet is resuspended in BRB80.<br> | |||
9. The final biotin-tubulin is frozen and stored as per the cycled | |||
tubulin.<br> | |||
</p> | </p> | ||
<p> | <p> | ||
=====Determination of stoichiometry===== | =====<font size="3">Determination of stoichiometry</font>===== | ||
Tubulin concentration was determined by SDS-PAGE electrophores.<br> | Tubulin concentration was determined by SDS-PAGE electrophores.<br> | ||
To quantify biotin, we use defference of avidin and avidin-biotin complex in <br> | |||
To quantify biotin, we use defference of avidin and avidin-biotin complex in | spectroscopic characteristic because avidin combines stoichiometrically with biotin.<br> | ||
The dye 4-hydroxyazobenzene-2’-carboxylic acid (HABA), which binds only to avidin with changing spectrum,<br> | |||
<br> | |||
spectroscopic characteristic because avidin combines stoichiometrically with | |||
biotin.<br> | |||
The dye 4-hydroxyazobenzene-2’-carboxylic acid (HABA), which binds only to | |||
avidin with changing spectrum,<br> | |||
so that it can be used as an indicator for unoccupied binding sites [1].<br> | so that it can be used as an indicator for unoccupied binding sites [1].<br> | ||
</p> | </p> | ||
<p> | <p> | ||
1. Prepare standard curve.<br> | <font size="3">1.Prepare standard curve.</font><br> | ||
---- | |||
Biotin standard solutions [(0, 10, 20, 50, 100, 200, 300, 500 µM) biotin,<br> | Biotin standard solutions [(0, 10, 20, 50, 100, 200, 300, 500 µM) biotin,<br> | ||
80 mM PIPES, 5 mM MgCl<sub>2</sub>, 1 mM EGTA] were prepared.<br> | 80 mM PIPES, 5 mM MgCl<sub>2</sub>, 1 mM EGTA] were prepared.<br> | ||
Each biotin standard solutions was added to avidin solution of which final | Each biotin standard solutions was added to avidin solution of which final concentration is<br> | ||
0.4 mg mL<sup>-1</sup> avidin, 250 mM HABA, 80 mM PIPES, 5 mM MgCl<sub>2</sub>, 1 mM EGTA.<br> | |||
concentration is<br> | |||
0.4 mg mL-1 avidin, 250 mM HABA, 80 mM PIPES, 5 mM MgCl<sub>2</sub>, 1 mM | |||
EGTA.<br> | |||
The mixtures were left for 15 min at roomtemperature.<br> | The mixtures were left for 15 min at roomtemperature.<br> | ||
A<sub>500</sub> of these solutions were measured to prepare standard curve. | |||
<br><br> | <br><br> | ||
2. Determination of biotin.<br> | <font size="3">2.Determination of biotin.</font><br> | ||
1 mg mL-1 Pronase was added to biotin-labeled tubulin, and left for 1 h at 37 | ---- | ||
1 mg mL<sup>-1</sup> Pronase was added to biotin-labeled tubulin, and left for 1 h at 37 °C.<br> | |||
This mixture was added to avidin solution, and left for 15 min at roomtemperature.<br> | |||
A<sub>500</sub> of this solution were measured to determine concentration of biotin.<br> | |||
====<font size="4">Preparation of Microtubule</font>==== | |||
Biotinylated- and rhodamine-labeled MTs were obtained by polymerizing biotin–tubulin <br> | |||
and rhodamine–tubulin (60% byotinilated and 10% rhodamine-tubulin; final tubulin concentration, 42 mM);<br> | |||
the solution containing the MTs was then diluted with motility buffer (80 mM PIPES, 1 mMEGTA,<br> | |||
2 mM MgCl<sub>2</sub>, 0.5 mg mL<sup>-1</sup> casein, 1 mM DTT, 4.5 mg mL<sup>-1</sup> D-glucose,<br> | |||
50 U mL<sup>-1</sup> glucose oxidase, 50 U mL<sup>-1</sup> catalase, 10 mM paclitaxel,<br> | |||
and 1% DMSO; pH 6.8). | |||
====<font size="4">Dynamic self-assembly</font>==== | |||
<p> | |||
Flow-cells were prepared by placing a coverglass on a slideglass (26 × 76 mm2) equipped with<br> | |||
a pair of double-sided tape to form a chamber of approximately 4 × 18 × 0.1 mm<sup>3</sup> (W × L × H) in dimension.<br> | |||
The flow cell was filled with 0.2 mg mL<sup>-1</sup> anti-GFP antibody (Invitrogen) for 3 min,<br> | |||
followed by a wash with 5 µL of casein solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl<sub>2</sub>, 0.5 mg mL<sup>-1</sup> casein; pH adjusted to 6.8 using HCl). <br> | |||
After incubating for 3 min with casein solution to mask the remaining glass surface, 5 µL of 200 nM kinesin solution <br> | |||
(80 mM PIPES, 40 mM NaCl, 1 mM EGTA, 1 mM MgCl<sub>2</sub>, 0.5 mg mL<sup>-1</sup> casein, 1 mM DTT, 4.5 mg mL<sup>-1</sup> D-glucose,<br> | |||
50 U mL<sup>-1</sup> glucose oxidase, 50 U mL<sup>-1</sup> catalase, 10 mM paclitaxel, 1% DMSO; pH 6.8) <br> | |||
were introduced and incubated for 3 min to bind the kinesins to the antibody. The flow cell<br> | |||
was washed with 3 mL of motility buffer. 3 mL of MTs solution (5000 nM in motility buffer, Bt 3000 nM) was then <br> | |||
introduced and incubated for 3 min, followed by washing with 5 µL of motility buffer.<br> | |||
A diluted solution (5 µL) of streptavidin (300 nM in motility buffer) was then introduced <br> | |||
and incubated for 3 min, followed by washing with 5 µL of motility buffer. Finally, Dynamic<br> | |||
self-assembly was initiated by applying 5 µL of ATP solution (motility buffer supplemented with 5 mM ATP). | |||
This experiment was performed at room temperature.<br> | |||
</p> | |||
<center> | |||
[[Image:リングの形成.png | 400px]] | |||
</center> | |||
<br><br><br><br> | |||
<br> | |||
<br> | |||
---- | ---- | ||
===[[Image:大見出し.png| 30px]] <font size="5">Preparation of Marimo-Gel</font>=== | |||
=====Preparation of spinach thylakoid membranes | ====[[Image:小見出し.png| 18px]] <font size="4">Preparation of spinach thylakoid membranes</font>==== | ||
<p> | <p> | ||
All purification procedures were performed at 4 °C. <br> | All purification procedures were performed at 4 °C. <br> | ||
Thylakoid membranes were prepared from spinach leaves by the modified method of | Thylakoid membranes were prepared from spinach leaves by the modified method of Yu et al.<br> | ||
The spinach leaves ( ~160 g) were washed with deionized water and homogenized in 500 mL of homogenization solution<br> | |||
Yu et al.<br> | (0.3 M sucrose, 20 mM NaCl, 5 mM MgCl<sub>2</sub>, 50 mM Tris-HCl, pH was adjusted to 7.6)<br> | ||
The spinach leaves ( | |||
500 mL of homogenization solution<br> | |||
(0.3 M sucrose, 20 mM NaCl, 5 mM MgCl<sub>2</sub>, 50 mM Tris-HCl, pH was | |||
adjusted to 7.6)<br> | |||
for 40 sec using an AM-10 homogenizer (Nihon Seiki Seisakusho, Japan). <br> | for 40 sec using an AM-10 homogenizer (Nihon Seiki Seisakusho, Japan). <br> | ||
The homogenate was filtered through four-time folded gauze.<br> | The homogenate was filtered through four-time folded gauze.<br> | ||
The flow through was suspended in 400 mL of a high ionic strength buffer (10 mM | The flow through was suspended in 400 mL of a high ionic strength buffer (10 mM Hepes-KOH, 150 mM NaCl, pH8).<br> | ||
The suspension was centrifuged at 10,000g for 20 min and the precipitate was resuspended in 15 mL<br> | |||
Hepes-KOH, 150 mM NaCl, pH8).<br> | of homogenization solution including 5% DMSO and flash frozen in liquid nitrogen,<br> | ||
The suspension was centrifuged at 10,000g for 20 min and the precipitate was | and stored in liquid nitrogen. | ||
resuspended in 15 mL<br> | |||
of homogenization solution including 5% DMSO and flash frozen in liquid | |||
nitrogen, and stored in liquid nitrogen. | |||
</p> | </p> | ||
<p>(Yu A. H. C; Hosono K. ''Biotechnol. Lett.'' '''1991''', ''13'', 411.)</p> | <p>(Yu A. H. C; Hosono K. ''Biotechnol. Lett.'' '''1991''', ''13'', 411.)</p> | ||
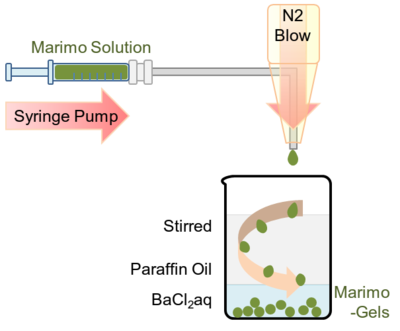
=====Entrapment of thylakoid membranes in alginate beads | ====[[Image:小見出し.png| 18px]] <font size="4">Entrapment of thylakoid membranes in alginate beads</font>==== | ||
<p> | <p> | ||
This procedure is based on the method described by Paul F. et al. and Zekorn T. | This procedure is based on the method described by Paul F. et al. and Zekorn T. et al.<br> | ||
A 0.3 mL of suspension of thylakoid membrane containing 1.41 mg protein/mL and 2.7 mL of<br> | |||
et al.<br> | a 2%(w/v) sodium alginate solution (100 mM K<sub>3</sub>PO<sub>4</sub>, 100 mM MgCl<sub>2</sub>, 100 mM NaCl, 500 mM PIPES,<br> | ||
A 0.3 mL of suspension of thylakoid membrane containing 1.41 mg protein/mL and | 200 mM ADP, pH was adjusted to 7.6) were placed in a 1 mL syringe. Using syringe pump (HA2000P, Harvard apparatus, US),<br> | ||
the alginate solution including thylakoid membrane was slowly ejected from the needle and was blown by a nitrogen gas.<br> | |||
2.7 mL of<br> | The tear shaped green droplet firstly encountered mineral oil phase and transformed into globular shape. <br> | ||
a 2%(w/v) sodium alginate solution (100 mM | Then the droplet sunk into the second phase, which contains 50 mM BaCl<sub>2</sub> and cross-linkage of <br> | ||
alginate with barium occurred. Because leak was not observed even after few weeks from the encapsulation,<br> | |||
mM PIPES,<br> | the cross-linked alginate mesh seemed to be enough small to support thylakoid membranes.<br> | ||
200 mM ADP, pH was adjusted to 7.6) were placed in a 1 mL syringe. Using syringe | |||
pump (HA2000P, Harvard apparatus, US),<br> | |||
the alginate solution including thylakoid membrane was slowly ejected from the | |||
needle and was blown by a nitrogen gas.<br> | |||
The tear shaped green droplet firstly encountered mineral oil phase and | |||
transformed into globular shape. <br> | |||
Then the droplet sunk into the second phase, which contains 50 mM | |||
BaCl<sub>2</sub> and cross-linkage of <br> | |||
alginate with barium occurred. Because leak was not observed even after few | |||
weeks from the encapsulation,<br> | |||
the cross-linked alginate mesh seemed to be enough small to support thylakoid | |||
membranes.<br> | |||
</p> | </p> | ||
<p> | <p> | ||
(Paul F.; Vignais P. M. ''Enzyme Mcrob. Technol.'' '''1980''', ''2'', 281.)<br> | (Paul F.; Vignais P. M. ''Enzyme Mcrob. Technol.'' '''1980''', ''2'', 281.)<br> | ||
(Zekron T.; Horcher A.; Siebers U.; Schnettler R.; Klock G.; Hering B.; | (Zekron T.; Horcher A.; Siebers U.; Schnettler R.; Klock G.; Hering B.; Zimmermann U.;<br> | ||
Zimmermann U.;<br> | |||
Bretzel R. G.; Federlin K. ''Acta Diabetol'', '''1992''', ''29'', 99.) | Bretzel R. G.; Federlin K. ''Acta Diabetol'', '''1992''', ''29'', 99.) | ||
</p> | </p> | ||
[[Image:marimogel.png|400px]] | |||
<br><br><br> | |||
---- | ---- | ||
===[[Image:大見出し.png| 30px]] <font size="5">Preparation of Micrometer-sized Gear</font>=== | |||
<p> | <p> | ||
First, Lift-off Layer (LOL) layer and SU-8 layer were placed on a silicon substrate. <br> | |||
Micro gears were drawn on SU-8 layer by using laser etching technique. Micro gears were<br> | |||
separated from silicon substrate by using alkaline developer (NMD-3) which can dissolve LOL layer. <br> | |||
After removal of micro gears from silicon substrate were collected by centrifugation.<br> | |||
</p> | |||
<center> | |||
[[Image:Gear.png| 500px]] | |||
</center> | |||
<br><br><br> | |||
===[[Image:大見出し.png| 30px]] <font size="5">Measurement the power of rotational motion of ring</font>=== | |||
<p> | |||
To measure the force of rotational motion of MT ring, we used optical tweezers. After MT ring preparation, we washed <br> | |||
the flow cell with motility buffer and put the polystyrene bead by biotin-streptavidin specific interaction. <br> | |||
Polystyrene bead was trapped with laser beam and measured the rotating power of MT ring. | |||
</p> | |||
<center> | |||
[[Image:Solution_2.png| 400px]] | |||
</center> | |||
samples but eliminated the UV portion of the radiation, thus minimizing the | ---- | ||
===[[Image:大見出し.png| 30px]] <font size="5">Devices</font>=== | |||
harmful effect of UV radiation on the samples.<br> | ====[[Image:小見出し.png| 18px]] <font size="4">Microscope</font>==== | ||
Moreover we connected a cooled-CMOS camera (NEO sCMOS, Andor) to a PC for | <p> | ||
To study the motility of MTs, we used a 100 W mercury lamp for illuminating of samples and<br> | |||
capturing images. | an epifluorescence microscope (Eclipse Ti, Nikon) using an oil-coupled Plan Apo 60×objective (Nikon)<br> | ||
for visualizing of samples. Also we used UV cut-off filter blocks (GFP-HQ: EX455-485, DM495, BA500-545; Nikon)<br> | |||
in the optical path of the microscope; these blocks allowed visualization of samples but eliminated the UV portion of the radiation, thus minimizing the harmful effect of UV radiation on the samples.<br> | |||
Moreover we connected a cooled-CMOS camera (NEO sCMOS, Andor) to a PC for capturing images. | |||
</p> | </p> | ||
<p> | <p> | ||
| Line 324: | Line 229: | ||
</p> | </p> | ||
=====Optical tweezer | ====[[Image:小見出し.png| 18px]] <font size="4">Optical tweezer</font>==== | ||
<p>Optical tweezer is a scientific instruments which can hold and move | <p>Optical tweezer is a scientific instruments which can hold and move microscopic objects <br> | ||
by using a highly focused laser beam. It provides an attractive or repulsive force (typically on the order of pN),<br> | |||
microscopic objects <br> | depending on the refractive index mismatch. In this work, we will measure the force of<br> | ||
by using a highly focused laser beam. It provides an attractive or repulsive | |||
force (typically on the order of pN),<br> | |||
depending on the refractive index mismatch. In this work, we will measure the | |||
force of<br> | |||
micro gear by using an optical tweezer.<br> | micro gear by using an optical tweezer.<br> | ||
Here we use a Nd:YAG laser (1064 nm wavelength) to trap a biological specimens. | Here we use a Nd:YAG laser (1064 nm wavelength) to trap a biological specimens. <br> | ||
This is because such specimens (being mostly water) have a low absorption coefficient at this wavelength. <br> | |||
<br> | A low absorption is desirable so as to minimize damage of the biological specimens, <br> | ||
This is because such specimens (being mostly water) have a low absorption | which sometimes referred to as opticution. Moreover He:Ne Pilot laser(633 nm wavelength) <br> | ||
coefficient at this wavelength. <br> | |||
A low absorption is desirable so as to minimize damage of the biological | |||
specimens, <br> | |||
which sometimes referred to as opticution. Moreover He:Ne Pilot laser(633 nm | |||
wavelength) <br> | |||
was used with Nd:YAG laser for visualization of specimens. <br> | was used with Nd:YAG laser for visualization of specimens. <br> | ||
Capturing images were sent to a PC and edited by imaging software (Nikon NIS | Capturing images were sent to a PC and edited by imaging software (Nikon NIS Elements).</p> | ||
<center> | |||
[[Image:Optical tweezer.jpg| 400px]] | [[Image:Optical tweezer.jpg| 400px]] | ||
</ | [[Image:Optical_tweezer_device.png| 400px]] | ||
</center> | |||
Latest revision as of 08:00, 31 August 2013
 Preparation of Ring-Shaped Microtubule Assemblies
Preparation of Ring-Shaped Microtubule Assemblies
 Purification of tubulin
Purification of tubulin
Tubulin was purified from porcine brain through two cycles of polymerization-depolymerization
using a high-molarity buffer (1 M PIPES, 20 mM EGTA, 10 mM MgCl2; pH adjusted to 6.8);
high-molarity PIPES buffer (HMPB).
1.Preparation of Brain homogenate
Porcine brains were purchased from a local slaughterhouse, and conserved before
use in ice-cold PBS (20 mM Na-phosphate, 150 mM NaCl, pH7.2). Brains were
cleaned of blood clots with , weighed, and transferred to a homogenizer (nissei, AM-7).
Cold (4 °C) depolymerization buffer (DB) (50 mM 2-[N-morpholino] ethanesulfonic acid,
1 mM CaCl2, pH 6.6) was added at a ratio of 1 mL/g of brain tissue. The mixture
was homogenized eight times at 50000 rpm for 15 s.
2.First cold and warm spin
Brain homogenates were then centrifuged at 12,000 rpm for 60 min at 4 °C.
The supernatants were pooled and supplemented with an equal volume of warm (37 °C)
high-molarity PIPES buffer (HMPB) (1 M PIPES, 10 mM MgCl2, and 20 mM EGTA, pH 6.9) ,
ATP (1.5 mM final), and GTP (0.5 mM final). ATP is included in this step to
remove motors and other proteins, which bind to microtubules in an ATP sensitive manner [2].
An equal volume (1/3 of the final volume) of pre-warmed to 37 °C anhydrous glycerol
was added to this solution. This mixture was incubated in a 37 °C water bath for 1h.
The polymerized tubulin was then centrifuged at 44,000 rpm for 30 min at 37 °C.
3.Second cold and warm spin
The resulting microtubule pellets were resuspended in 100 mL of cold DB.
The depolymerized tubulin was subsequently centrifuged in at 34,000 rpm for 30 min at 4 °C.
The supernatant from this centrifugation step was mixed with an equal volume
of the HMPB supplemented with ATP and GTP, followed by the addition of glycerol
(1/3 of final volume) as described above. The mixture was incubated in a 37 °C
water bath for 1h, and the polymerized microtubules centrifuged in at 44,000 rpm
for 30 min at 37 °C. Following the centrifugation, the microtubule pellets were resuspended
in 15 mL of ice-cold BRB80 (80 mM PIPES, 1 mM MgCl2, 1 mM EGTA, pH 6.8) and then
incubated for a further 10 min on ice. After this polymerization step, the tubulin
was centrifuged in at 35,000 rpm for 30 min at 4 °C. The supernatant was collected
and snap-frozen in 100 µL aliquots in liquid nitrogen.
4.Determination of tubulin concentration
Tubulin concentration was determined by SDS-PAGE.
 Preparation of biotin labeled tubulin
Preparation of biotin labeled tubulin
1. Tubulin was thawed, and polymerized at 37 °C for 15 min.
2. Biotin-XX-SE (Molecular Probes, Cat. B-1606) was dissolved at 0.1 M in dry dimethyl sulfoxide (DMSO).
3. Biotin-XX-SE solution was added to tubulin, while pipetting to distribute it rapidly to a final concentration of 2 mM. incubate at 37 °C for 20 min.
4. Mixture was layered onto cushions and spun at 54,000 rpm for 1h at 37 °C.
5. Pellets were resuspended and spun cold, being careful to wash the cushion inter face well to remove all the biotin-XX-SE.
6. Tubulin was depolymerized and spun 40,000 rpm for 15 min at 4 °C.
7. Steps (3) – (6) were performed to give once cycled biotin-tubulin.
8. Steps (3) – (6) were repeated to give twice cycled biotin-tubulin. The final pellet is resuspended in BRB80.
9. The final biotin-tubulin is frozen and stored as per the cycled tubulin.
Determination of stoichiometry
Tubulin concentration was determined by SDS-PAGE electrophores.
To quantify biotin, we use defference of avidin and avidin-biotin complex in
spectroscopic characteristic because avidin combines stoichiometrically with biotin.
The dye 4-hydroxyazobenzene-2’-carboxylic acid (HABA), which binds only to avidin with changing spectrum,
so that it can be used as an indicator for unoccupied binding sites [1].
1.Prepare standard curve.
Biotin standard solutions [(0, 10, 20, 50, 100, 200, 300, 500 µM) biotin,
80 mM PIPES, 5 mM MgCl2, 1 mM EGTA] were prepared.
Each biotin standard solutions was added to avidin solution of which final concentration is
0.4 mg mL-1 avidin, 250 mM HABA, 80 mM PIPES, 5 mM MgCl2, 1 mM EGTA.
The mixtures were left for 15 min at roomtemperature.
A500 of these solutions were measured to prepare standard curve.
2.Determination of biotin.
1 mg mL-1 Pronase was added to biotin-labeled tubulin, and left for 1 h at 37 °C.
This mixture was added to avidin solution, and left for 15 min at roomtemperature.
A500 of this solution were measured to determine concentration of biotin.
Preparation of Microtubule
Biotinylated- and rhodamine-labeled MTs were obtained by polymerizing biotin–tubulin
and rhodamine–tubulin (60% byotinilated and 10% rhodamine-tubulin; final tubulin concentration, 42 mM);
the solution containing the MTs was then diluted with motility buffer (80 mM PIPES, 1 mMEGTA,
2 mM MgCl2, 0.5 mg mL-1 casein, 1 mM DTT, 4.5 mg mL-1 D-glucose,
50 U mL-1 glucose oxidase, 50 U mL-1 catalase, 10 mM paclitaxel,
and 1% DMSO; pH 6.8).
Dynamic self-assembly
Flow-cells were prepared by placing a coverglass on a slideglass (26 × 76 mm2) equipped with
a pair of double-sided tape to form a chamber of approximately 4 × 18 × 0.1 mm3 (W × L × H) in dimension.
The flow cell was filled with 0.2 mg mL-1 anti-GFP antibody (Invitrogen) for 3 min,
followed by a wash with 5 µL of casein solution (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein; pH adjusted to 6.8 using HCl).
After incubating for 3 min with casein solution to mask the remaining glass surface, 5 µL of 200 nM kinesin solution
(80 mM PIPES, 40 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 0.5 mg mL-1 casein, 1 mM DTT, 4.5 mg mL-1 D-glucose,
50 U mL-1 glucose oxidase, 50 U mL-1 catalase, 10 mM paclitaxel, 1% DMSO; pH 6.8)
were introduced and incubated for 3 min to bind the kinesins to the antibody. The flow cell
was washed with 3 mL of motility buffer. 3 mL of MTs solution (5000 nM in motility buffer, Bt 3000 nM) was then
introduced and incubated for 3 min, followed by washing with 5 µL of motility buffer.
A diluted solution (5 µL) of streptavidin (300 nM in motility buffer) was then introduced
and incubated for 3 min, followed by washing with 5 µL of motility buffer. Finally, Dynamic
self-assembly was initiated by applying 5 µL of ATP solution (motility buffer supplemented with 5 mM ATP).
This experiment was performed at room temperature.
 Preparation of Marimo-Gel
Preparation of Marimo-Gel
 Preparation of spinach thylakoid membranes
Preparation of spinach thylakoid membranes
All purification procedures were performed at 4 °C.
Thylakoid membranes were prepared from spinach leaves by the modified method of Yu et al.
The spinach leaves ( ~160 g) were washed with deionized water and homogenized in 500 mL of homogenization solution
(0.3 M sucrose, 20 mM NaCl, 5 mM MgCl2, 50 mM Tris-HCl, pH was adjusted to 7.6)
for 40 sec using an AM-10 homogenizer (Nihon Seiki Seisakusho, Japan).
The homogenate was filtered through four-time folded gauze.
The flow through was suspended in 400 mL of a high ionic strength buffer (10 mM Hepes-KOH, 150 mM NaCl, pH8).
The suspension was centrifuged at 10,000g for 20 min and the precipitate was resuspended in 15 mL
of homogenization solution including 5% DMSO and flash frozen in liquid nitrogen,
and stored in liquid nitrogen.
(Yu A. H. C; Hosono K. Biotechnol. Lett. 1991, 13, 411.)
 Entrapment of thylakoid membranes in alginate beads
Entrapment of thylakoid membranes in alginate beads
This procedure is based on the method described by Paul F. et al. and Zekorn T. et al.
A 0.3 mL of suspension of thylakoid membrane containing 1.41 mg protein/mL and 2.7 mL of
a 2%(w/v) sodium alginate solution (100 mM K3PO4, 100 mM MgCl2, 100 mM NaCl, 500 mM PIPES,
200 mM ADP, pH was adjusted to 7.6) were placed in a 1 mL syringe. Using syringe pump (HA2000P, Harvard apparatus, US),
the alginate solution including thylakoid membrane was slowly ejected from the needle and was blown by a nitrogen gas.
The tear shaped green droplet firstly encountered mineral oil phase and transformed into globular shape.
Then the droplet sunk into the second phase, which contains 50 mM BaCl2 and cross-linkage of
alginate with barium occurred. Because leak was not observed even after few weeks from the encapsulation,
the cross-linked alginate mesh seemed to be enough small to support thylakoid membranes.
(Paul F.; Vignais P. M. Enzyme Mcrob. Technol. 1980, 2, 281.)
(Zekron T.; Horcher A.; Siebers U.; Schnettler R.; Klock G.; Hering B.; Zimmermann U.;
Bretzel R. G.; Federlin K. Acta Diabetol, 1992, 29, 99.)
 Preparation of Micrometer-sized Gear
Preparation of Micrometer-sized Gear
First, Lift-off Layer (LOL) layer and SU-8 layer were placed on a silicon substrate.
Micro gears were drawn on SU-8 layer by using laser etching technique. Micro gears were
separated from silicon substrate by using alkaline developer (NMD-3) which can dissolve LOL layer.
After removal of micro gears from silicon substrate were collected by centrifugation.
 Measurement the power of rotational motion of ring
Measurement the power of rotational motion of ring
To measure the force of rotational motion of MT ring, we used optical tweezers. After MT ring preparation, we washed
the flow cell with motility buffer and put the polystyrene bead by biotin-streptavidin specific interaction.
Polystyrene bead was trapped with laser beam and measured the rotating power of MT ring.
 Devices
Devices
 Microscope
Microscope
To study the motility of MTs, we used a 100 W mercury lamp for illuminating of samples and
an epifluorescence microscope (Eclipse Ti, Nikon) using an oil-coupled Plan Apo 60×objective (Nikon)
for visualizing of samples. Also we used UV cut-off filter blocks (GFP-HQ: EX455-485, DM495, BA500-545; Nikon)
in the optical path of the microscope; these blocks allowed visualization of samples but eliminated the UV portion of the radiation, thus minimizing the harmful effect of UV radiation on the samples.
Moreover we connected a cooled-CMOS camera (NEO sCMOS, Andor) to a PC for capturing images.
 Optical tweezer
Optical tweezer
Optical tweezer is a scientific instruments which can hold and move microscopic objects
by using a highly focused laser beam. It provides an attractive or repulsive force (typically on the order of pN),
depending on the refractive index mismatch. In this work, we will measure the force of
micro gear by using an optical tweezer.
Here we use a Nd:YAG laser (1064 nm wavelength) to trap a biological specimens.
This is because such specimens (being mostly water) have a low absorption coefficient at this wavelength.
A low absorption is desirable so as to minimize damage of the biological specimens,
which sometimes referred to as opticution. Moreover He:Ne Pilot laser(633 nm wavelength)
was used with Nd:YAG laser for visualization of specimens.
Capturing images were sent to a PC and edited by imaging software (Nikon NIS Elements).