20.109(S07): Bio-material engineering/Optimizing panning
Introduction
Thanks to recent advances in DNA synthesis chemistry, high quality pools of oligonucleotides are available at reasonable concentrations and prices. A quick look around the Internet for companies performing DNA synthesis (companies such as Integrated DNA Technologies or Operon) shows that it’s possible to get next day delivery on a 20 or 30 base sequence of your choosing for around 20 dollars. The synthesis chemistry requires only a few things: a solid support on which to build the oligos, nucleotides that can be protected from further nucleotide additions, a way of deprotecting the nucleotides once they’ve been added to the oligo chain, and finally a way of releasing the finished oligos from the support at the end of the synthesis. An automated synthesizer and a schematic of synthesis chemistry are shown.

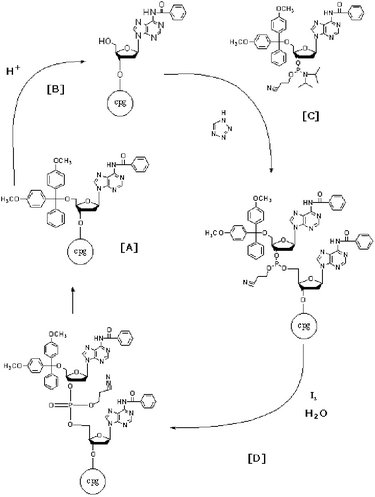
Consider making a 4-mer: GATC. To begin the synthesis, the 3’ hydroxyl group of the first nucleotide (guanosine) would be coupled to a 500 angstrom bead (”cpg”). The guanosine would then be treated with a solution (dichloroacteic acid) to remove the cap (a dimethoxytrityl group) protecting its 5’ phosphate. The phosphate can then react with the 3’ hydroxyl of the incoming nucleotide (adenosine), which would also be capped on its 5’ end to prevent the addition of multiple adenines to the growing chain. Unincorporated adenine would be washed away before the adenine on the bound GA-dinucleotide was deprotected and reacted with a protected thymidine. Next, the GAT-trinucleotide would be washed to remove unincorporated T, deprotected and reacted with cytosine. Ultimately, the final 4-mer would be released from the solid support to be further purified by chromatography or electrophoresis if needed.
The same chemistry was used to make the library you've been working with but at each nucleotide addition step, an equimolar mixture of all four bases was reacted generating a pool of random DNAs. After uncoupling the oligos from the solid support, the pool was made double stranded with a DNA polymerase and then inserted into the pCT-CON plasmid, with each plasmid getting one version of the oligo. Consequently, the junction of the Aga2 sequence with the 36-base pair oligo is the same in every plasmid but the 12 amino acid sequence fused to each Aga2 protein can be different. Some of these will bind gold and most will not.
You have already screened the library once to isolate some gold-binders from the mix. Your results might resemble the following.
Digital Photographs of Gold Slides after Panning



Yeast Eluted from Slides, growing as colonies on selective media



Sensibly enough, the pAu1 panning gave the most colonies because every yeast from that pool can bind gold. Interestingly, more yeast were isolated from the library pool than from the negative control, a hopeful indication that some novel gold-binding yeast have been identified. Do all these library candidates bind gold equally well? Unlikely. The affinity will depend on the sequence of the Aga2 fusion protein and each yeast colony from the library could have a different sequence. In fact, some of the candidates may not really bind gold at all. For example cells that were trapped on the glass behind the gold slide would appear to have bound the gold in your initial screen. Today you will choose four library candidates to further evaluate next time, re-examining their gold-binding ability and determining their relative affinity.
Most of your time today will be spent repeating the gold panning from last time, changing one parameter that may improve the protocol. Consider the contents of the solutions you used, the times and temperatures for the binding and washing steps and the mechanics of the yeast-gold incubation. Any one of these can be modified today.
Protocol
Part 1: Optimizing panning conditions
The only thing real ground rule here is that everyone will compare pCT-CON and pAu1. What experimental condition you vary in the panning protocol is up to you. The drawing below can be used to sketch your experimental set up. It may be possible for you to work on the other parts of today's experiments while the yeast are incubating with the gold.

Part 2: Library rescreen
Examine the Petri dishes that you spread last time. Count the number of colonies on each plate and enter your data in the table on the discussion page for this lab. It is OK to count only 1/2 or 1/4 or 1/8 of the colonies if they are densely growing. In these cases, try to find a representative sector to count and don’t forget to multiply for the total number of colonies on the plate. Circle four colonies that are nicely isolated from others on the "library" petri dish. Label those colonies "A" "B" "C" or "D".
Next you should set up four tubes with 2.5 ml of glucose-containing media and four tubes with 5 ml of galactose-containing media. Use colored dots to label the tops of each “A” “B” “C” or “D.” Use sterile technique since these will be used to grow any gold binding candidates for next time.
Finally use a sterile dowel to transfer some of the correct colony from your petri dish to the glucose-containing media, and balance the tubes on the roller wheel in the 30° incubator. Give the galactose-containing media to the teaching faculty who will innoculate them before next lab.You should also return to the teaching faculty the petri dish from the library screen so it can be stored until next time.
Part 3: Research proposal
Writing a research proposal requires that you identify an interesting topic, spend lots of time learning about it, and then design some clever experiments to advance the field. It also requires that you articulate your ideas so any reader is convinced of your expertise, your creativity and the significance of your findings, should you have the opportunity to carry out the experiments you’ve proposed. To begin you must identify your research question. This may be the hardest part and the most fun. Fortunately you started by finding a handful of topics to share with your lab partner. Today you should discuss and evaluate the topics you’ve gathered. Consider them based on:
- your interest in the topic
- the availability of good background information
- your likelihood of successfully advancing current understanding
- the possibility of advancing foundational technologies or finding practical applications
- if your proposal could be carried out in a reasonable amount of time and with non-infinite resources
It might be that not one of the topics you’ve identified is really suitable, in which case you should find some new ideas. It’s also possible that through discussion with your lab partner, you’ve found something new to consider. Both of these outcomes are fine but by the end of today’s lab you should have settled on a general topic or two so you can begin the next step in your proposal writing, namely background reading and critical thinking about the topic.
A few ground rules that are BE.109 specific:
- you should not propose any research question that has been the subject of your UROP or research experience outside of BE.109. This proposal must be original.
- you should keep in mind that this proposal will be presented to the class, so try to limit your scope to an idea that can be convincingly presented in a ten minute oral presentation.
Once you and your partner have decided on a suitable research problem, it’s time to become an expert on the topic. This will mean searching the literature, talking with people, generating some ideas and critically evaluating them. To keep track of your efforts, you should start a wiki catalog on your OpenWetWare user page. How you format the page is up to you but check out the “yeast rebuild” or the “T7.2” wiki pages on OpenWetWare for examples of research ideas in process. As part of your “for next time assignment” you will have to print out your wiki page specifying your topic, your research goal and at least five helpful references that you’ve read and summarized.
DONE!
For next time
- To better understand how the library of plasmids was synthesized, please review the chemistry involved in joining nucleotides during DNA replication. Draw the addition of a guanine nucleotide to a thymidine dimer, clearly indicating the 5’ and 3’ end.
- Prepare a table presenting the plating results of your gold binding experiment from last time as well as a short description of the experiment to go with the table.
- Define your research proposal by making a wiki page to collect your ideas and resources (you can do this on one page with your partner or split the effort and each turn in an individual page). Keep in mind that your presentation to the class will need:
- a brief project overview
- sufficient background information for everyone to understand your proposal
- a statement of the research problem and goals
- project details and methods
- predicted outcomes if everything goes according to plan and if nothing does
- needed resources to complete the work
You can organize your wiki page along these lines or however you feel is most helpful. Print your user page(s) for next time, making sure it defines your topic, your idea and some references you've collected and summarized.
