20.109(S07): cDNA synthesis and microarray: Difference between revisions
No edit summary |
No edit summary |
||
| Line 58: | Line 58: | ||
#Heat your hybridization solutions to 80° for 10 minutes then cool them to room temperature. During this time you will be shown how to assemble the hybridization chambers. | #Heat your hybridization solutions to 80° for 10 minutes then cool them to room temperature. During this time you will be shown how to assemble the hybridization chambers. | ||
#Load your samples into the hybridization chambers and tape them with your colored tape. Two groups will share one slide since each slide has two arrays.Put today’s date on the tape. When every group is ready, we will walk the arrays over to the BioMicroCenter in Building 68 to put them in the 60°C hybridization oven. | #Load your samples into the hybridization chambers and tape them with your colored tape. Two groups will share one slide since each slide has two arrays.Put today’s date on the tape. When every group is ready, we will walk the arrays over to the BioMicroCenter in Building 68 to put them in the 60°C hybridization oven. | ||
====Here’s what will happen tomorrow:==== | |||
*One of the teaching faculty will wash the unbound cDNAs off your arrays. The wash steps will be 6xSSC/0.005% Triton X-100 at 60° then 2X SSC/0.005% Triton X-100 at 60° for 15 minutes then 2X SSC at room temperature for 10 minutes then 0.1X SSC at room temperature for 10 minutes. | |||
*The slides will be dried then rehybridized with the Cy3 and Cy5 agents at 60°C for four hours. Genisphere sells these agents as “dendrimers,” essentially large fluorescent balls with an average of 850 fluorophores each. These are described in detail at http://www.genisphere.com/about_3dna.html | |||
During this time, the Cy3 and Cy5 agents will bind the capture sequences on the cDNAs bound to the arrays. | |||
*After 4 hours, the unbound Cy3 and Cy5 agents will be washed off your arrays. The wash steps will be 2X SSC/0.005% Triton X-100 at 60°C for 15 minutes, 2X SSC at room temperature for 10 minutes and 0.1X SSC at room temperature for 10 minutes. The slides will be dried very quickly using Nitrogen gas to blow off any water droplets, then scanned in the Agilent scanner that is available in the BioMicroCenter. The data from your array will be available for you to analyze next time. | |||
DONE! | DONE! | ||
==For next time== | ==For next time== | ||
==Reagents list== | ==Reagents list== | ||
*cDNA synthesis cocktail from Genisphere | |||
**Superscript First Strand Buffer | |||
**DTT | |||
**Superase-In | |||
**dNTPs | |||
**Superscript II enzyme | |||
*2X Hybridization Buffer from Agilent | |||
*20X SSC from Ambion | |||
**3M NaCl | |||
**0.3 M NaCitrate | |||
Revision as of 05:15, 31 December 2006
Introduction
Today you will use one tool, a DNA microarray, to simultaneously examine the expression of many genes. DNA microarrays are slides with DNA sequences spotted in a known order on the surface. The spots of DNA, each one smaller than the period at the end of this sentence, are placed on the slide surface with robotic arms or built one base at a time with photolithography. Each spot of DNA gets a unique address on the slide surface, and the identity and location of each spot get stored in the computerized “design file” for the array. The slide shown below is the same size as the one you’ll use (1 x 3 inches) but yours will have 11,000 spots of DNA arrayed instead of the 250 shown!

Two spots on the illustrated array are highlighted. The first spot, in Row 6 Position 30, is a 60-nucleotide sequence from the human gene for glyceraldehyde-3-phosphate dehydrogenase (GAPD). This gene, which encodes an essential metabolic enzyme, has been called a “housekeeping” gene since it must be expressed in all cells no matter how specialized. Other housekeeping genes include those for ACTB (encoding a cytoskeletal protein), TBP (encoding a general transcription factor), HPRT (encoding an enzyme required for nucleotide transport and metabolism), and PPIA (encoding an enzyme important for protein folding). The second spot highlighted on the array, in Row 4 Position 10, is a 60-nucleotide sequence from the human TERT gene. This gene encodes the protein subunit of telomerase, an enzyme that adds telomere repeats (TTGGGGTTG) to the end of chromosomes. As healthy cells age and divide, telomere repeats are lost. Cancerous cells express telomerase and so the telomeres do not shorten. Consequently, these cells “lose track” of how old they are and become immortal.
The GAPD and TERT spots can be used to illustrate how microarray data is generated and interpreted. Consider a group of “normal” cells and a cancerous version of them. RNA from each type of cell can be isolated (you’ve seen how quick and easy it is to isolate RNA), converted from RNA into a complementary strand of DNA (called cDNA), and then “color coded.” The most commonly used molecules for color-coding are the green-fluorescing cyanine 3 (Cy3) and the red-fluorescing cyanine 5 (Cy5).
For this example, the normal cells get green and the cancerous cells get red. The two colored samples are mixed and then simultaneously hybridized to a DNA microarray. The DNA spotted on the surface of the slide is in vast excess to either colored cDNA sample and so the intensity of each color will vary with the amount of RNA originally present in each sample. A gene expressed similarly in normal and cancerous cells, like the housekeeping GAPD gene, will give rise to a yellow spot in Row 6 Position 30 since equal amounts of green and red cDNA will be bound there and the merged color will appear yellow. By contrast, only red cDNA will bind at Row 4 Position 10 since cancerous cells express telomerase and normal cells do not.
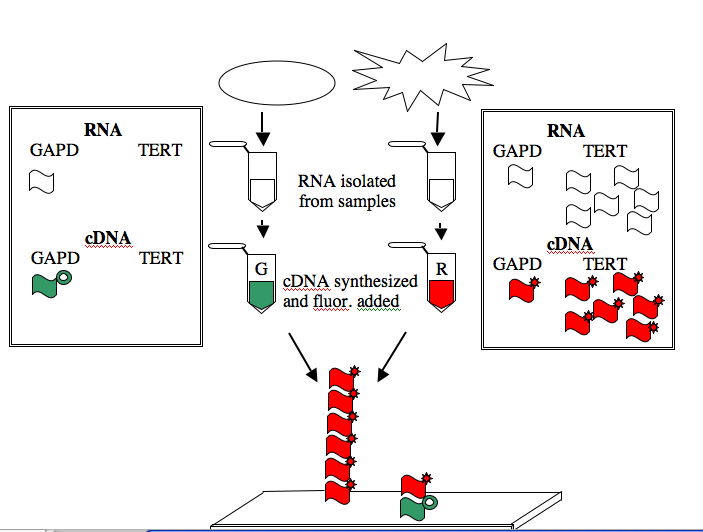
NOTE: The cDNAs are not really piled on top of one another on the array. Rather they are hybridized side by side to the spot of DNA that is on the surface of the slide.
With an expensive machine, the slide is “scanned” to measure the intensity of the red and green light at each spot (remember we’re talking about 22,000 spots!) and the data can then be assessed and normalized. Corrections are often made to account for differences between Cy3 and Cy5 incorporation into the cDNA as well as how much of each fluorescent molecule sticks non-specifically to different areas of the slide. These are things you will do next time with your own data.
Protocols
Today you will convert the RNA isolated from your transfected cells into cDNA and hybridize the cDNA to a DNA microarray.
Part 1: cDNA synthesis
Creating cDNA from RNA is done using an enzyme called reverse transcriptase. Like all DNA polymerases, this enzyme can only add sequence to an existing chain and so needs a short “primer” to begin synthesis. To perform the cDNA synthesis, you will use a kit from a company named Genisphere. The primers in this kit have a special “capture sequence” at their 5’ end. The capture sequences allow the cDNA to be reacted with Cy3 or Cy5 later.
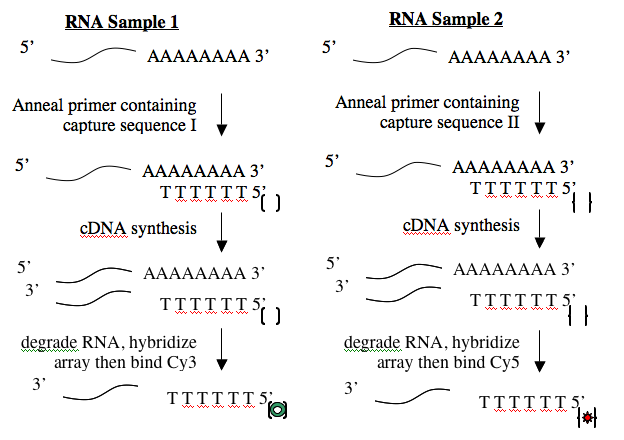
Before you begin today’s protocol, prepare your bench for working with RNA. This involves cleaning your pipetmen, retrieving your “RNA only” pipet tips and solutions and wiping down your bench. You should work on a fresh piece of benchpaper and remember to wear gloves when working with RNA.
Calculate the volume needed for 3.5 ug of each RNA sample, then assemble the annealing reactions in RNase-free eppendorf tubes according to the following table.
| TUBE A | TUBE B | |
|---|---|---|
| RNA | 3.5 ug of RNA from parental strain | 3.5 ug of RNA from deletion strain |
| RNase-free H20 | bring volume to 10 ul | bring volume to 10 ul |
| RT primer | 1 ul Capture Sequence I vial 11, red |
1 ul Capture Sequence II vial 11, blue |
- Heat the annealing reactions to 80°C for 10 minutes then place the tubes on ice for 2 minutes.
- Microfuge the tubes briefly to spin any condensation or droplets down to the bottom of the tube then add 9 ul of cDNA synthesis cocktail. Because reverse transcriptase is an unstable enzyme, this cocktail must be prepared just before use. The teaching faculty will prepare some for you when you are ready for it.
- Pipet the contents of your tubes up and down to gently mix, then incubate the cDNA synthesis reactions at 42° for 1.5 hours. During this time, work with the sample array data file that is available (see Part 2 of today’s protocol).
- Microfuge the tubes briefly then add 3.5 ul of 0.5M NaOH/0.5M EDTA to each tube and pipet up and down to mix. Heat to 65°C for 10 minutes. This step will denature your RNA/cDNA hybrids and degrade the RNA.
- Add 5 ul of 1M Tris, pH7 to neutralize the contents of each tube.
Part 2: practice array data analysis
Part 3: hybridize microarrays
The arrays we will use are the yeast v2 DNA microarrays from Agilent. Each slide has two arrays , each with 11,000 60-mer oligonucleotides. These “oligos” were first built on glass wafers and then printed onto the slide surface. The oligos represent more than 6000 yeast genes, many spotted on the slide more than once.
The success of your experiment is absolutely dependent on the following:
- You must hold the slides by the edges only. If you touch the array that is printed on the slide’s surface, you will obscure the DNA that is printed there.
- Each array has a barcode printed on some stickers on one end of each slide. The array is printed on the side of the slide that says “Agilent.” If you try to hybridize your cDNA to the numbered side of the slide, there will be no array there to bind.
to hybridize the arrays
- Begin by mixing the cDNA pools you have synthesized into one eppendorf tube. The total volume should be 57 ul. Add 198 ul of H2O and 255 ul of 2X Hybridization Buffer. Pipet up and down several times to mix the contents.
- Heat your hybridization solutions to 80° for 10 minutes then cool them to room temperature. During this time you will be shown how to assemble the hybridization chambers.
- Load your samples into the hybridization chambers and tape them with your colored tape. Two groups will share one slide since each slide has two arrays.Put today’s date on the tape. When every group is ready, we will walk the arrays over to the BioMicroCenter in Building 68 to put them in the 60°C hybridization oven.
Here’s what will happen tomorrow:
- One of the teaching faculty will wash the unbound cDNAs off your arrays. The wash steps will be 6xSSC/0.005% Triton X-100 at 60° then 2X SSC/0.005% Triton X-100 at 60° for 15 minutes then 2X SSC at room temperature for 10 minutes then 0.1X SSC at room temperature for 10 minutes.
- The slides will be dried then rehybridized with the Cy3 and Cy5 agents at 60°C for four hours. Genisphere sells these agents as “dendrimers,” essentially large fluorescent balls with an average of 850 fluorophores each. These are described in detail at http://www.genisphere.com/about_3dna.html
During this time, the Cy3 and Cy5 agents will bind the capture sequences on the cDNAs bound to the arrays.
- After 4 hours, the unbound Cy3 and Cy5 agents will be washed off your arrays. The wash steps will be 2X SSC/0.005% Triton X-100 at 60°C for 15 minutes, 2X SSC at room temperature for 10 minutes and 0.1X SSC at room temperature for 10 minutes. The slides will be dried very quickly using Nitrogen gas to blow off any water droplets, then scanned in the Agilent scanner that is available in the BioMicroCenter. The data from your array will be available for you to analyze next time.
DONE!
For next time
Reagents list
- cDNA synthesis cocktail from Genisphere
- Superscript First Strand Buffer
- DTT
- Superase-In
- dNTPs
- Superscript II enzyme
- 2X Hybridization Buffer from Agilent
- 20X SSC from Ambion
- 3M NaCl
- 0.3 M NaCitrate
