20.109(S08):Prepare expression system (Day3): Difference between revisions
| Line 140: | Line 140: | ||
==For next time== | ==For next time== | ||
#Plot your fluorescence data against an arbitrary ''x''-axis (such as 1, 2, 3, ... 12) and describe the shape. Where does the inflection point(s) of the curve fall with respect to the calcium solutions 1-12 (whose exact concentrations will remain unidentified for now). | |||
#The vector [https://catalog.invitrogen.com/index.cfm?fuseaction=viewCatalog.viewProductDetails&productDescription=615 pRSET] has several properties that make it useful for protein expression and production in bacteria. Some of these were described in today’s Introduction. Name 2 other features contained in the pRSET vector and what purposes they serve. <br> | #The vector [https://catalog.invitrogen.com/index.cfm?fuseaction=viewCatalog.viewProductDetails&productDescription=615 pRSET] has several properties that make it useful for protein expression and production in bacteria. Some of these were described in today’s Introduction. Name 2 other features contained in the pRSET vector and what purposes they serve. <br> | ||
#BL21(DE3) ''E. coli'' are often used for protein expression. In contrast, XL1-Blue ''E. coli'' are ‘workhorse’ cells useful for plasmid propagation. What are the two modified genes in XL1-Blue that make them ideal for this task? It may help you to refer to the cell manual: [http://www.stratagene.com/products/displayProduct.aspx?pid=548 (pdf download)]. | #BL21(DE3) ''E. coli'' are often used for protein expression. In contrast, XL1-Blue ''E. coli'' are ‘workhorse’ cells useful for plasmid propagation. What are the two modified genes in XL1-Blue that make them ideal for this task? It may help you to refer to the cell manual: [http://www.stratagene.com/products/displayProduct.aspx?pid=548 (pdf download)]. | ||
Revision as of 15:40, 21 January 2008
Introduction
Now that we have prepared DNA encoding your mutant inverse pericams, we would like to actually produce the physical proteins. Last time you were here, you prepared mutagenized DNA from a template plasmid. Your oh-so devoted teaching staff transformed this DNA into competent XL1-Blue cells, like the ones you used in Module 1. Mutant colonies were picked the next day for liquid overnight cultures, which in turn were miniprepped to purify mutant plasmid DNA. However, the inverse pericam protein itself cannot be produced by XL1-Blue cells. Today, you will transform your IPC mutant plasmids into a bacterial system that can produce the protein directly.
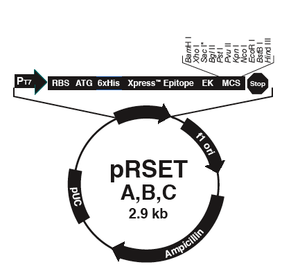
The bacterial expression vector we are using (pRSET) contains the bacteriophage T7 promoter. This promoter is active only in the presence of T7 RNA polymerase (T7RNAP), an enzyme that therefore must be expressed by the bacterial strain used to make the protein of interest. We will use the BL21(DE3)pLysS strain, which has the following genotype: F-, ompT hsdSB (rB- mB-) gal dcm (DE3) pLysS (CamR). In BL21(DE3), T7RNAP is associated with a lac construct, and its expression is under the control of the lacUV5 promoter. Due to the action of the lac repressor (lacI gene), the polymerase will not be produced except in the presence of lactose or a small-molecule lactose analogue such as IPTG (isopropyl β-D-thiogalactoside). To further reduce ‘leaky’ expression of the protein of interest (in our case, inverse pericam), the pLysS version of BL21(DE3) contains T7 lysozyme, which also inhibits basal transcription of T7RNAP. This gene is retained by Chloramphenicol selection, while the pRSET plasmid itself (and thus inverse pericam) is retained by Ampicillin selection.

In order to transform BL21(DE3) cells with your mutant IPC plasmids, you will first have to make the cells competent, i.e., able to efficiently take up foreign DNA. In Module 1, we used commercially available competent cells that did not need further treatment prior to DNA addition. Today, you will make chemically competent cells using calcium chloride, then incubate them with plasmid DNA and heat shock them as before prior to plating. Tomorrow, the teaching staff will pick colonies and set up liquid overnight cultures from your transformed cells. Next time, you will add IPTG to these liquid cultures to induce expression of your mutant proteins, which you will then isolate and characterize. Much of this process is summarized in the figure at left.
To get a sense for what this characterization will entail, today you will obtain a titration curve against calcium for wild-type inverse pericam (like the one in the paper by Nagai et al. that we discussed last time) using a benchtop fluorimeter. Our particular piece of equipment, called a Nanodrop, has the distinct advantage of requiring only very small volumes of protein per measurement. (Why might this be useful?) However, each sample is measured manually, and the sample holder must be cleaned between each use. Many bench-top protocols can be scaled up to high-throughput assays. In general, what kind of assay you decide to perform will depend on how many samples you expect to test, the convenience of equipment access (if it is in a multi-user facility), the costs associated with the equipment, and the relative accuracy and precision of the assays. Later in the term, when you are measuring several tens of samples instead of a mere dozen, we will use an automated fluorescence plate reader, and revisit the topic of scale.
Protocols
Part 1: prepare competent BL21(DE3) cells
- Pick up two 3 mL tubes of BL21(DE3) cells. These cells should be in or close to the mid-log phase of growth, which is indicated by an OD600 value of 04.-06.
- Measure the OD600 value of a 1:10 dilution of your cells. If the cells are not yet dense enough, return them to the rotary shaker in the incubator. Remember to balance your tubes! As a rule, your cells should double every 20-30 min.
- Once your cells have reached the appropriate growth phase, spin down 4 tubes of ~ 1.5 mL each, aspirate the supernatants, and resuspend in an equal volume of ice-cold calcium chloride (100 mM).
- Spin again for 1 min. The resultant pellets should occur as streaks down the side of the eppendorf tube, so be very careful not to disturb the cells when aspirating.
- This time, resuspend each pellet in 100 μL of CaCl2, then pool the cells together in one tube.
- Incubate on ice for 1 hour. (You might work on parts 3-5 of today's protocols now.)
- Meanwhile, label five eppendorfs (for four transformations and a no DNA control) and pre-chill them on ice. (You can label your mutant tubes as X#Z, where # is the residue number you are modifying, X is the original amino acid, and Z is the mutant acid. The example shown on Day 1 would be Y64D.)
Part 2: transform BL21(DE3) with mutant DNA
- Prewarm and dry ten LB+Amp/Cam plates by placing them in the 37°C incubator, media side up with the lids ajar. You will perform two transformations for each of your five samples – one with a concentrated cell stock, and one with a 1:10 dilution of cells.
- When your competent cells are ready, aliquot 75 μL of cells per pre-chilled eppendorf.
- Add 2 μL of the appropriate DNA to each tube. Remember, you are testing plasmid DNA that was prepared from two different colonies for each of your two mutants, along with a no DNA control.
- Flick to mix the contents and leave the tubes on ice for at least 5 minutes.
- Heat shock the cells at 42°C for 90 seconds exactly. Use your timer.
- Move the samples to a rack on your bench then use your P1000 to add 0.5 ml of LB media to each eppendorf tube. Invert each tube to mix.
- Incubate the tubes in the 37°C incubator for at least 30 minutes. This gives the antibiotic-resistance genes some time be expressed in the transformed bacterial cells.
- While you are waiting, prepare 4 large glass test tubes containing LB+Amp/Cam, and label them with your team color and sample name.
- Also prepare 5 eppendorf tubes containing 180 μL of LB each. Use these to dilute your transformed cells 1:10 when you retrieve them from the incubator.
- Plate 200 μl of each transformation mix on an LB+Amp/Cam plate. Make sure to label which concentration of cells was used on each plate. Safety reminder: After dipping the glass spreader in the ethanol jar, then pass it through the flame of the alcohol burner just long enough to ignite the ethanol. After letting the ethanol burn off, the spreader may still be very hot, and it is advisable to tap it gently on a portion of the agar plate without cells in order to equilibrate it with the agar (if it sizzles, it's way too hot).
- Once the plates are done, wrap them with colored tape and incubate them in the 37°C incubator overnight. One of the teaching faculty will remove them from the incubator and set up liquid cultures for you to use next time.
Part 3: titration curve for wild-type protein, round 1
- You will prepare and measure 12 samples. For each sample, mix 10 μL of protein solution with 10 μL of the appropriate calcium solution (obtain aliquots from the teaching faculty). Store on ice until use.
- Attach the Nanodrop to the USB port (in the back) of your computer.
- Double-click on the ND-3300 icon to start the software.
- Click on ‘Other Fluorophores’ and select ‘FITC-FixedGain’ from the menu.
- You should automatically be prompted for a blank (i.e., background) solution. You will blank on an aliquot of the buffer labeled EB.
- Lift the Nanodrop arm, add 2 μL of blanking solution atop the lower platform, and gently replace the arm. You do not need to push the arm down for the sensors to meet. The Nanodrop will do this automatically when it makes its reading.
- Click on the ‘Blank’ button and wait for the reading to be completed.
- Lift the arm, then wipe both the lower platform and the upper bushing with a chemical wipe.
- Put 2 μL of blanking solution on the sensor, close the arm, and this time hit ‘Measure’ instead of ‘Blank.’ If you have a relatively flat line at ~0 RFU (relative fluorescence units), proceed to the first sample. Otherwise, wipe the platform and bushing several times and try again.
- For each sample, place 2 μL of solution on the platform, then gently lower the arm and hit ‘Measure’. You should see a single peak centered ~ 515 nm.
- If you see erratic behaviour, clean the Nanodrop by wiping thoroughly with chemical tissue, and rinsing (2 μL at a time) with protein-free buffer. In fact, you might measure your blanking solution in between each sample, to ensure that you are having minimal carryover of solution from sample-to-sample.
- As you proceed with each sample, you can write in the Sample ID, then save and print out your data at the end (use the Show Report function). You can also simply fill in the table below by hand.
| Sample | Intensity (515 nm) | Sample | Intensity (515 nm) |
|---|---|---|---|
| 1 | 7 | ||
| 2 | 8 | ||
| 3 | 9 | ||
| 4 | 10 | ||
| 5 | 11 | ||
| 6 | 12 |
Hang on to this data until Day 7 for a complete analysis. For now, you will just make a few qualitative notes about your data in today’s homework assignment.
Part 4: count mutant colonies
When you have a spare moment today, count the colonies that arose on each of your transformed XL1-Blue plates. Do the control samples have no colonies? Do the two mutations appear to have different efficiencies?
Part 5: Prepare sequencing reactions
As we will discuss in lab today, sequencing reactions require a primer for initiation. Legible readout of the gene typically begins about 40-50 bp downstream of the primer site, and continues for ~1000 bp at most. Thus, multiple primers must be used to fully view genes > 1 Kbp in size. How many basepairs long is inverse pericam? (Try using the Word Count feature on the the sequence document.)
The recommended composition of sequencing reactions is 200-500 ng of plasmid DNA and 3.2 pmoles of sequencing primer in a final volume of 12 μL. You will prepare two reactions per candidate (8 reactions total), one with a forward-reading primer and one with a reverse primer. Do not mix the primers together in one tube! The miniprep'd plasmid should have ~1 μg of nucleic acid/μL but that will be a mixture of RNA and DNA, so we will guess at the amount of plasmid DNA appropriate for our reactions.
For each reaction, combine the following reagents in an eppendorf tube:
- 2 μL of your plasmid DNA candidate
- 5.3 μL of a 1:100 dilution of the sequencing primer
- 12.7 μL sterile water
For each solution, mix by pipetting and then transfer 12 μL to an 8-PCR-tube strip. Keep track of which sample is in which tube (A-H), and label your tubes according to the table below. The teaching faculty will turn in the strips at the Biopolymers Laboratory in E17 for sequencing.
| Group | Label Range |
|---|---|
| Green | 1-8 |
| Purple | 9-16 |
| Red | 17-24 |
| Pink | 25-32 |
| Blue | 33-40 |
| Yellow | 41-48 |
For next time
- Plot your fluorescence data against an arbitrary x-axis (such as 1, 2, 3, ... 12) and describe the shape. Where does the inflection point(s) of the curve fall with respect to the calcium solutions 1-12 (whose exact concentrations will remain unidentified for now).
- The vector pRSET has several properties that make it useful for protein expression and production in bacteria. Some of these were described in today’s Introduction. Name 2 other features contained in the pRSET vector and what purposes they serve.
- BL21(DE3) E. coli are often used for protein expression. In contrast, XL1-Blue E. coli are ‘workhorse’ cells useful for plasmid propagation. What are the two modified genes in XL1-Blue that make them ideal for this task? It may help you to refer to the cell manual: (pdf download).
