20.109(S13):DNA cloning (Day4): Difference between revisions
| (19 intermediate revisions by the same user not shown) | |||
| Line 32: | Line 32: | ||
===Part 1: Gel electrophoresis of PCR products=== | ===Part 1: Gel electrophoresis of PCR products=== | ||
You will use a 1% agarose gel to run your three PCRs from last time, as well as a reference lane of molecular weight markers (also called a DNA ladder). | You will use a 1.5% agarose gel to run your three PCRs from last time, as well as a reference lane of molecular weight markers (also called a DNA ladder). | ||
#Add | #Add 2.5 μL of loading dye to each of three eppendorf tubes. No need to change tips in between. | ||
#*Loading dye contains xylene cyanol as a tracking dye to follow the progress of the electrophoresis (so you don’t run the smallest fragments off the end of your gel!) as well as glycerol to help the samples sink into the well. | #*Loading dye contains xylene cyanol as a tracking dye to follow the progress of the electrophoresis (so you don’t run the smallest fragments off the end of your gel!) as well as glycerol to help the samples sink into the well. | ||
#Now add | #Now add 20 μL of each reaction from last time (your sample, your partner's sample, and the no template control) to individual eppendorf tubes and label them. | ||
#Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom. | #Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom. | ||
#*Remember: to quick-spin, hold down the "short" button on your centrifuge for 3-5 seconds, then release. | #*Remember: to quick-spin, hold down the "short" button on your centrifuge for 3-5 seconds, then release. | ||
#Load the gel according to the table below. Up to 4 groups will share each gel: 2 groups per lane. | #Load the gel according to the table below. Up to 4 groups will share each gel: 2 groups per lane. | ||
#*To load your samples, draw | #*To load your samples, draw 20 μL into the tip of your P20. Lower the tip below the surface of the buffer and directly over the well. You risk puncturing the bottom of the well if you lower the tip too far into the well itself (puncturing well = bad!). Slowly expel your sample into the well. Do not release the pipet plunger until after you have removed the tip from the gel box (or you'll draw your sample back into the tip!). | ||
#Once all the samples have been loaded, we will attach the gel box to the power supply and run at 110 V for 45 minutes. | #Once all the samples have been loaded, we will attach the gel box to the power supply and run at 110 V for 45 minutes. | ||
#Later you will be shown how to photograph your gel and excise the relevant | #Later you will be shown how to photograph your gel and, if necessary, to excise the relevant band of DNA. Begin by anticipating where you expect to see your sample band relative to the bands of the DNA ladder, [http://www.neb.com/nebecomm/products/productN3231.asp described here]. | ||
[[Image:Mod1 2 agaro gel.jpg|thumb|right|200px|'''Loading a gel''']] | [[Image:Mod1 2 agaro gel.jpg|thumb|right|200px|'''Loading a gel''']] | ||
| Line 50: | Line 48: | ||
{| border="1" | {| border="1" | ||
! Lane | ! Lane | ||
! Sample ( | !Sample (20 μL) | ||
!Lane | !Lane | ||
!Sample ( | !Sample (20 μL) | ||
|- | |- | ||
| 1 | | 1 | ||
| Line 83: | Line 81: | ||
<br style="clear:both;"/> | <br style="clear:both;"/> | ||
<font color = CC0000>'''For ease of comparing which PCR yielded more product for identical starting samples, Groups 1 and 2 should couple as: Red with Blue, Orange with Pink, Yellow with Purple, and Green with Platinum.'''</font color> | |||
===Part 1B: OPTIONAL -- Purify 16S PCR band from gel=== | ===Part 1B: OPTIONAL -- Purify 16S PCR band from gel=== | ||
In many types of cloning, PCR products are purified prior to ligation. For the cloning approach that we are taking, the manufacturer recommends using PCR products directly. Indeed, in pilot experiments, we found that cloning was at least as successful with direct PCR products as | In many types of cloning, PCR products are purified prior to ligation. For the cloning approach that we are taking, the manufacturer recommends using PCR products directly. Indeed, in pilot experiments, we found that cloning was at least as successful with direct PCR products as with gel-purified PCR products. However, if your PCR resulted in multiple bright bands on your agarose gel, then you will need to isolate the 16S band before proceeding. | ||
In gel purification, a DNA band is melted, then isolated on a silica (SiO<sub>2</sub>) column similar to the one you used last time. Salt concentration and pH effects, along with ethanol precipitation, will alternately allow for binding and eluting the DNA while washing away contaminants. The protocol is linked [[20.109(S13):Optional gel purification | '''separately here''']] to avoid clutter on this page. | In gel purification, a DNA band is melted, then isolated on a silica (SiO<sub>2</sub>) column similar to the one you used last time. Salt concentration and pH effects, along with ethanol precipitation, will alternately allow for binding and eluting the DNA while washing away contaminants. The protocol is linked [[20.109(S13):Optional gel purification | '''separately here''']] to avoid clutter on this page. | ||
===Part 2: Ligate purified product to vector arms=== | ===Part 2: Ligate purified product to vector arms=== | ||
#For today, you will work with a temporary lab partner! First, choose whichever PCR yielded a brighter band on the gel (assuming no non-specific products in either), and perform '''one''' ligation and transformation together. Ultimately, you will each prepare your own plate of cells. '''Please save both extra PCR reactions and leftover ligation mix today. These will be stored frozen in case of later needs.''' | |||
#*For example, if Shannon and Agi both worked with gull sample 842, and Shannon's band was brighter, then Shannon and Agi would perform the procedure below with Shannon's PCR, but they wouldn't throw away Agi's PCR either. | |||
#Prepare a clearly labeled eppendorf tube for | #Prepare a clearly labeled eppendorf tube for the cloning reaction. | ||
#In the following order, combine 3 μL cloning buffer, 2 μL | #In the following order, combine 3 μL cloning buffer, 2 μL DNA, and 1 μL of vector mix. | ||
#Incubate at room temperature for 5 min, and then move to ice if you are not yet ready to proceed with the next step. Meanwhile, obtain an aliquot of competent cells from the teaching faculty and let them slowly thaw on ice. Label the side of | #Incubate at room temperature for 5 min, and then move to ice if you are not yet ready to proceed with the next step. Meanwhile, obtain an aliquot of competent cells from the teaching faculty and let them slowly thaw on ice -- takes about 5 min, in fact. Label the side of the tube (and also the top if you like). | ||
#When both cells and reaction are ready, add 1 μL of the reaction to the cells. Do not pipet up-and-down more than once! Instead, ''gently'' mix the cells and DNA by twirling the tube in your fingers. | #When both cells and reaction are ready, add 1 μL of the reaction to the cells. Do not pipet up-and-down more than once! Instead, ''gently'' mix the cells and DNA by twirling the tube in your fingers. | ||
#*Hold the tube near the top, so that the cells at the bottom stay cold. | #*Hold the tube near the top, so that the cells at the bottom stay cold. | ||
| Line 103: | Line 103: | ||
#Add 250 μL of warm LB to each sample; do not pipet to mix. | #Add 250 μL of warm LB to each sample; do not pipet to mix. | ||
#Place the tubes on the nutator in the 37 °C incubator, and rock them for about 1 hr. | #Place the tubes on the nutator in the 37 °C incubator, and rock them for about 1 hr. | ||
#*You may want to put a colored sticky label on | #*You may want to put a colored sticky label on the tube so you can quickly identify it later. | ||
# | #Shortly after starting your incubation, pick up two LB-Amp plates from the incubator and bring them to the front teaching bench. A member of the teaching faculty will demonstrate how to spread 40 μL of X-gal onto the plates. Return your well-labeled plates (team/day/initials/sampleID/date) to the incubator to warm up further. | ||
===Part 3: Prepare tubes for liquid O/N cultures=== | ===Part 3: Prepare tubes for liquid O/N cultures=== | ||
| Line 116: | Line 116: | ||
===Part 4: Transform ligated product into cloning strain=== | ===Part 4: Transform ligated product into cloning strain=== | ||
#Pick up an ethanol jar, spreader, lighter, and alcohol burner when you are almost ready to begin. Don't forget to wear your safety glasses! | #Pick up an ethanol jar, spreader, lighter, and alcohol burner when you are almost ready to begin. Don't forget to wear your safety glasses! | ||
#Plate 100 μL of each transformation mix onto an LB-Amp-Xgal plate. After dipping the glass spreader in the ethanol jar, you should pass it through the flame of the alcohol burner '''just long enough to ignite the ethanol'''. '''After letting the ethanol burn off, the spreader may still be very hot''', and it is advisable to tap it gently on a portion of the agar plate without cells in order to equilibrate it with the agar (if it sizzles, it's way too hot). Once the plates are ready, | #Plate 100 μL of each transformation mix onto an LB-Amp-Xgal plate. After dipping the glass spreader in the ethanol jar, you should pass it through the flame of the alcohol burner '''just long enough to ignite the ethanol'''. '''After letting the ethanol burn off, the spreader may still be very hot''', and it is advisable to tap it gently on a portion of the agar plate without cells in order to equilibrate it with the agar (if it sizzles, it's way too hot). Once the plates are ready, place them in the 37°C incubator overnight; be sure that each is labeled with your (original) team color and gull sample ID. One of the teaching faculty will remove them from the incubator and set up liquid cultures for you to use next time. | ||
===Part 5: Prepare primers for microsporidia PCR=== | ===Part 5: Prepare primers for microsporidia PCR=== | ||
| Line 124: | Line 123: | ||
We will run your microsporidia PCRs in bulk sometime before Day 5, after preparing a large master mix that includes polymerase and template(s) directly. We'll expect you to prepare primer stocks for us today. Steps: | We will run your microsporidia PCRs in bulk sometime before Day 5, after preparing a large master mix that includes polymerase and template(s) directly. We'll expect you to prepare primer stocks for us today. Steps: | ||
# | #Calculate the amount of water needed ''for each primer'' (forward and reverse, separately) to give a concentration of 100 μM. | ||
#Touch-spin your primers, resuspend each in the appropriate volume of sterile water, and touch-spin again. | |||
#Now prepare and intermediate dilution from your archival stock. Prepare 100 μL of a solution that has ''each'' primer present at 2 μM. | |||
#*Try the calculation on your own first... trying... trying... you should have come up with two microliters of each primer plus ninety-six microliters of water. | |||
#*Be sure to change tips between primers! | |||
#Return the rest of your primers, plus your primer specification sheets, up front. | |||
For your reference, the PCR conditions will be... [write on Day 5 instead?] | For your reference, the PCR conditions will be... [write on Day 5 instead?] | ||
| Line 170: | Line 174: | ||
==For next time== | ==For next time== | ||
'''Parts 2, 3, and 4 of this assignment are also due on Stellar.''' | |||
#Prepare the overview schematic of your experimental approach that will be included in the Introduction or (more likely) at the beginning of the Results. | #Prepare the overview schematic of your experimental approach that will be included in the Introduction or (more likely) at the beginning of the Results. | ||
#*Recall that our collaboration and integrity guidelines state that your text and figures must be your own unless otherwise specified. Thus, you should all make your own schematic rather than modifying one from class slides or the wiki. Keep in mind that you are being evaluated not on advanced PowerPoint skills, but instead on showing that you understand the purpose and major steps of the experiment and can convey them. | #*Recall that our collaboration and integrity guidelines state that your text and figures must be your own unless otherwise specified. Thus, you should all make your own schematic rather than modifying one from class slides or the wiki. Keep in mind that you are being evaluated not on advanced PowerPoint skills, but instead on showing that you understand the purpose and major steps of the experiment and can convey them. | ||
#and | #Write the opening context-setting paragraph of your Results section, in which you introduce the system you are working with and your immediate investigative goal. | ||
#*This paragraph might be somewhat longer or shorter depending on the level of detail in your Introduction section, but it cannot be omitted entirely. | |||
#Draft an outline of the rest of the results to indicate the approximate high-level content and its order. You don't need to state the actual results (in fact, you don't have them all yet!), just each ''type'' of result that will be discussed in about a sentence each. Essentially, what order will your figures/tables/data be in and what will they consist of? Also include sub-section titles in this draft outline. | |||
#Prepare a figure depicting your PCR gel results (from today) with an appropriate caption. Also write the portion of your Results (likely one rather short paragraph) describing this experiment. | |||
==Reagent list== | ==Reagent list== | ||
| Line 191: | Line 200: | ||
*100 bp DNA ladder from New England BioLabs | *100 bp DNA ladder from New England BioLabs | ||
*Qiagen QIAquick gel extraction kit | |||
*StrataClone Blunt PCR Cloning kit from Agilent | |||
**Includes StrataClone SoloPack competent cells | |||
*Luria-Bertani broth from Teknova | |||
**1% Tryptone | |||
**0.5 % Yeast extract | |||
**1 % NaCl | |||
*2% X-gal, aka 5-bromo-4-chloro-3-inodlyl-β-D- galactopyranoside | |||
**original 5% stock in dimethylformamide (DMF), diluted to 2% in LB | |||
*Optional: Qiagen QIAquick gel extraction kit | |||
**silica spin columns | **silica spin columns | ||
**QG and PE buffers | **QG and PE buffers | ||
*Isopropanol | **Isopropanol | ||
<font color=red>added later</font color> | |||
*Ampicillin: 100 mg/mL, aqueous, sterile-filtered | |||
* | *LB+AMP plates | ||
** | **LB with 2% agar and 100 μg/ml Ampicillin | ||
**Later, 40 μL of 2% X-gal was spread on each plate | |||
Latest revision as of 14:55, 4 March 2013
Introduction
Welcome back! Last time you performed PCR on the DNA pool you extracted from a gull sample. Specifically, you used primers designed to amplify bacterial 16S rRNA genes from this complex pool. Now you want to systematically analyze individual fragments from this 16S DNA pool, which is aided by a process called cloning.
In traditional cloning, an insert is ligated to a backbone to create a circular piece of DNA called a vector. Typically, the insert and backbone are cut with special proteins called restriction enzymes (more about those in Module 2!) that yield complementary overhangs at the ends of the two pieces of DNA. The enzyme DNA ligase then chemically bonds the insert and backbone together by catalyzing formation of a phosphodiester bond. Ligated vectors are selected in bacteria by including an antibiotic resistance marker on the backbone, and growing the bacteria in said antibiotic. (We’ll say more about that procedure below.) This method is especially suitable for cloning with the purpose of creating a well-defined piece of DNA.

When diverse products must be cloned for subsequent analysis, as in our case, we would like to omit some steps from the above workflow. A few methods exist for cloning PCR products directly. The most popular approach is dubbed TA cloning, and it exploits the fact that polymerases such as Taq add an adenine to the 3’ end of PCR products. This single-base overhang alone can be used in a process very much like traditional cloning. Recall, however, that we used Pfu polymerase precisely for its precision, to improve our chance of successful amplification from a “difficult” sample such as stool. Pfu has proofreading capabilities, meaning that it doesn’t add spurious adenines.
Pieces of DNA that do not share complementary overhangs, that indeed have no overhangs at all but instead have blunt ends, can also be ligated together. Here the biology of DNA topoisomerase I is exploited. The backbone includes a CCCTT sequence, which is cut by the topoisomerase, resulting in so-called two vector arms. The vector arms remain bound to the topoisomerase, and can ligate back to each other or to a newly introduced insert sequence. The reaction mixture – buffer, vector arms, and insert (in our case the PCR from M1D3) – is then added to bacteria.
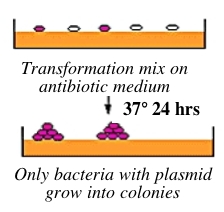
Bacteria can take up foreign DNA in a process called transformation, during which a single plasmid enters a bacterium and, once inside, replicates and expresses the genes it encodes. Most bacteria do not exist in a transformation-ready state, but can be made permeable to foreign DNA -- by chemical or other means -- and are then termed competent. After transformation, the bacteria are plated on an antibiotic-containing agar medium. In your case, the vector arms have an ampicillin resistance gene, and thus only bacteria that took up the vector can grow on ampicillin-containing plates, while untransformed cells will die before they can form a colony. Notice that a vector-containing bacterium will grow whether or not that vector contains a PCR product insert.
We would rather not waste our time analyzing colonies that contain pure vector DNA! Thus, the reaction mixture is added to a particular strain of bacteria that has a mutation in a metabolic gene, but also a complementation sequence that counteracts the mutation (called lacZ '). These bacteria can be grown on a special medium that results in a blue hue to the cell colonies. If an insert is ligated to the vector arms, the complementation is disrupted, and colonies appear white (or sometimes light blue) in hue due to the dysfunctional gene. Specifically, the mutant enzyme cannot cleave the substrate that is present in the media, and thus no colored product is formed.
One final piece of engineering that makes this particular blunt-end cloning approach possible is the use of bacteria that express Cre recombinase. The action of this enzyme, which recombines DNA at particular target sequences on the vector arms (loxP sites), is what makes the linear DNA (vector arm-insert-vector arm) into a circular plasmid that can be readily replicated in and isolated from bacteria. The cloning process as a whole is summarized in the figure below.

So today you’ll ligate and transform your PCR product pool, but only after visualizing that everything looks as expected on an agarose gel. Next time you will isolate DNA clones from individual colonies and send them off for sequencing. Then we’ll be just one step away from knowing what bacteria (a sampling of them, in any case!) are present in each gull sample.
Protocols
Part 1: Gel electrophoresis of PCR products
You will use a 1.5% agarose gel to run your three PCRs from last time, as well as a reference lane of molecular weight markers (also called a DNA ladder).
- Add 2.5 μL of loading dye to each of three eppendorf tubes. No need to change tips in between.
- Loading dye contains xylene cyanol as a tracking dye to follow the progress of the electrophoresis (so you don’t run the smallest fragments off the end of your gel!) as well as glycerol to help the samples sink into the well.
- Now add 20 μL of each reaction from last time (your sample, your partner's sample, and the no template control) to individual eppendorf tubes and label them.
- Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom.
- Remember: to quick-spin, hold down the "short" button on your centrifuge for 3-5 seconds, then release.
- Load the gel according to the table below. Up to 4 groups will share each gel: 2 groups per lane.
- To load your samples, draw 20 μL into the tip of your P20. Lower the tip below the surface of the buffer and directly over the well. You risk puncturing the bottom of the well if you lower the tip too far into the well itself (puncturing well = bad!). Slowly expel your sample into the well. Do not release the pipet plunger until after you have removed the tip from the gel box (or you'll draw your sample back into the tip!).
- Once all the samples have been loaded, we will attach the gel box to the power supply and run at 110 V for 45 minutes.
- Later you will be shown how to photograph your gel and, if necessary, to excise the relevant band of DNA. Begin by anticipating where you expect to see your sample band relative to the bands of the DNA ladder, described here.

| Lane | Sample (20 μL) | Lane | Sample (20 μL) |
|---|---|---|---|
| 1 | DNA ladder (load 10 μL) | 6 | DNA ladder (load 10 μL) |
| 2 | Group 1, NTC | 7 | Group 2, NTC |
| 3 | Group 1, sample A | 8 | Group 2, sample A |
| 4 | Group 1, sample B | 9 | Group 2, sample B |
| 5 | BLANK | 10 | BLANK |
For ease of comparing which PCR yielded more product for identical starting samples, Groups 1 and 2 should couple as: Red with Blue, Orange with Pink, Yellow with Purple, and Green with Platinum.
Part 1B: OPTIONAL -- Purify 16S PCR band from gel
In many types of cloning, PCR products are purified prior to ligation. For the cloning approach that we are taking, the manufacturer recommends using PCR products directly. Indeed, in pilot experiments, we found that cloning was at least as successful with direct PCR products as with gel-purified PCR products. However, if your PCR resulted in multiple bright bands on your agarose gel, then you will need to isolate the 16S band before proceeding.
In gel purification, a DNA band is melted, then isolated on a silica (SiO2) column similar to the one you used last time. Salt concentration and pH effects, along with ethanol precipitation, will alternately allow for binding and eluting the DNA while washing away contaminants. The protocol is linked separately here to avoid clutter on this page.
Part 2: Ligate purified product to vector arms
- For today, you will work with a temporary lab partner! First, choose whichever PCR yielded a brighter band on the gel (assuming no non-specific products in either), and perform one ligation and transformation together. Ultimately, you will each prepare your own plate of cells. Please save both extra PCR reactions and leftover ligation mix today. These will be stored frozen in case of later needs.
- For example, if Shannon and Agi both worked with gull sample 842, and Shannon's band was brighter, then Shannon and Agi would perform the procedure below with Shannon's PCR, but they wouldn't throw away Agi's PCR either.
- Prepare a clearly labeled eppendorf tube for the cloning reaction.
- In the following order, combine 3 μL cloning buffer, 2 μL DNA, and 1 μL of vector mix.
- Incubate at room temperature for 5 min, and then move to ice if you are not yet ready to proceed with the next step. Meanwhile, obtain an aliquot of competent cells from the teaching faculty and let them slowly thaw on ice -- takes about 5 min, in fact. Label the side of the tube (and also the top if you like).
- When both cells and reaction are ready, add 1 μL of the reaction to the cells. Do not pipet up-and-down more than once! Instead, gently mix the cells and DNA by twirling the tube in your fingers.
- Hold the tube near the top, so that the cells at the bottom stay cold.
- Incubate the mixture on ice for 20 min.
- Place the tubes in the 42 °C heat block for exactly 45 seconds, and transfer immediately immediately to ice for 2 min more.
- Add 250 μL of warm LB to each sample; do not pipet to mix.
- Place the tubes on the nutator in the 37 °C incubator, and rock them for about 1 hr.
- You may want to put a colored sticky label on the tube so you can quickly identify it later.
- Shortly after starting your incubation, pick up two LB-Amp plates from the incubator and bring them to the front teaching bench. A member of the teaching faculty will demonstrate how to spread 40 μL of X-gal onto the plates. Return your well-labeled plates (team/day/initials/sampleID/date) to the incubator to warm up further.
Part 3: Prepare tubes for liquid O/N cultures
You will make your teaching faculty very happy if you contribute to their preparatory work.
- Please label 8 large glass test tubes with your team color and sample ID, per sample.
- Mix 25 mL LB with 25 μL of ampicillin.
- Using a serological pipet, aliquot 2.5 mL of LB+Amp per tube. These will be used to set up liquid overnight cultures from your 8 colonies for next time.
Part 4: Transform ligated product into cloning strain
- Pick up an ethanol jar, spreader, lighter, and alcohol burner when you are almost ready to begin. Don't forget to wear your safety glasses!
- Plate 100 μL of each transformation mix onto an LB-Amp-Xgal plate. After dipping the glass spreader in the ethanol jar, you should pass it through the flame of the alcohol burner just long enough to ignite the ethanol. After letting the ethanol burn off, the spreader may still be very hot, and it is advisable to tap it gently on a portion of the agar plate without cells in order to equilibrate it with the agar (if it sizzles, it's way too hot). Once the plates are ready, place them in the 37°C incubator overnight; be sure that each is labeled with your (original) team color and gull sample ID. One of the teaching faculty will remove them from the incubator and set up liquid cultures for you to use next time.
Part 5: Prepare primers for microsporidia PCR
We will run your microsporidia PCRs in bulk sometime before Day 5, after preparing a large master mix that includes polymerase and template(s) directly. We'll expect you to prepare primer stocks for us today. Steps:
- Calculate the amount of water needed for each primer (forward and reverse, separately) to give a concentration of 100 μM.
- Touch-spin your primers, resuspend each in the appropriate volume of sterile water, and touch-spin again.
- Now prepare and intermediate dilution from your archival stock. Prepare 100 μL of a solution that has each primer present at 2 μM.
- Try the calculation on your own first... trying... trying... you should have come up with two microliters of each primer plus ninety-six microliters of water.
- Be sure to change tips between primers!
- Return the rest of your primers, plus your primer specification sheets, up front.
For your reference, the PCR conditions will be... [write on Day 5 instead?]
Most concentrations similar except primers at...
| Segment | Cycles | Temperature (° C) | Time |
|---|---|---|---|
| 1 | 1 | 95 | 3 min |
| 2-4 | 40 | 95 | 1 min |
| 58 | 1.5 min | ||
| 72 | 2 min | ||
| 5 | 1 | 72 | 10 min |
| 6 | 1 | 4 | indefinite |
For next time
Parts 2, 3, and 4 of this assignment are also due on Stellar.
- Prepare the overview schematic of your experimental approach that will be included in the Introduction or (more likely) at the beginning of the Results.
- Recall that our collaboration and integrity guidelines state that your text and figures must be your own unless otherwise specified. Thus, you should all make your own schematic rather than modifying one from class slides or the wiki. Keep in mind that you are being evaluated not on advanced PowerPoint skills, but instead on showing that you understand the purpose and major steps of the experiment and can convey them.
- Write the opening context-setting paragraph of your Results section, in which you introduce the system you are working with and your immediate investigative goal.
- This paragraph might be somewhat longer or shorter depending on the level of detail in your Introduction section, but it cannot be omitted entirely.
- Draft an outline of the rest of the results to indicate the approximate high-level content and its order. You don't need to state the actual results (in fact, you don't have them all yet!), just each type of result that will be discussed in about a sentence each. Essentially, what order will your figures/tables/data be in and what will they consist of? Also include sub-section titles in this draft outline.
- Prepare a figure depicting your PCR gel results (from today) with an appropriate caption. Also write the portion of your Results (likely one rather short paragraph) describing this experiment.
Reagent list
- Agarose gels
- Standard agarose prepared in TAE buffer at 1%
- With SYBR Safe stain (Invitrogen)
- used at manufacturer's recommended concentration, 10000-fold dilution
- Loading dye
- 0.25% xylene cyanol
- 30% glycerol
- RNase
- Gels made and run in 1X TAE buffer
- 40 mM Tris
- 20 mM Acetic Acid
- 1 mM EDTA, pH 8.3
- 100 bp DNA ladder from New England BioLabs
- StrataClone Blunt PCR Cloning kit from Agilent
- Includes StrataClone SoloPack competent cells
- Luria-Bertani broth from Teknova
- 1% Tryptone
- 0.5 % Yeast extract
- 1 % NaCl
- 2% X-gal, aka 5-bromo-4-chloro-3-inodlyl-β-D- galactopyranoside
- original 5% stock in dimethylformamide (DMF), diluted to 2% in LB
- Optional: Qiagen QIAquick gel extraction kit
- silica spin columns
- QG and PE buffers
- Isopropanol
added later
- Ampicillin: 100 mg/mL, aqueous, sterile-filtered
- LB+AMP plates
- LB with 2% agar and 100 μg/ml Ampicillin
- Later, 40 μL of 2% X-gal was spread on each plate
