20.109: 2-component signaling: Difference between revisions
No edit summary |
No edit summary |
||
| (27 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
===Module for F'12=== | |||
Mutagenesis of T541 | |||
===Oligos for screen=== | |||
*'''NO304''': K+P- screen of T541, mutagenesis oligo: <br> | |||
5'--CTG GCG GAT GAC CGC ACG CTG CTG ATG GCG GGG GTA AGT CAC GAC TTG CGC <font color = red>'''NNY'''</font color> CCG CTG ACG CGT ATT CGC CTG GCG ACT GAG ATG ATG -3' | |||
*'''NO305''': complementary oligo that binds to 3' end of mutagenic oligo and builds plasmid around the horn: | |||
5'- CAT CAT CTC AGT CGC CAG GCG AAT ACG CGT CAG -3' | |||
===Module for F'11=== | |||
*Remade reporter strain NB462 and tested for induction--using antibiotics at: Amp25, Cam34, Kan10, and including Kan10 always. | |||
*Remade library K+P- and K-P+. Used H557A as control for K-P+ screen | |||
*Retested indicator media. Phenol red failed, but Z4 gave clear colonies in dark and red colonies in light (except where dense). | |||
==Students will try to identify K-P+ mutants that appear more red in light, i.e. lower units in light, then check to see if dynamic range improved or if all activity diminished== | |||
*Day 1: set up dark/light system in plates, liq culture | |||
*Day 2: b-gal assay of cultures, set up photo | |||
*Day 3: transform library and screen, recapitulate setup electronically | |||
*Day 4: Journal club I, re-streak candidate, <teacher ONs in light and dark> | |||
*Day 5: DNA for seq, and b-gal assay | |||
*Day 6: Protein gel and blot, seq data, photo?, other assays | |||
*Day 7: Probe blot, other assays | |||
*Day 8: Journal club, data back | |||
====Oligos ordered for F'11 screen==== | |||
*'''NO294''': K-P+ library, mutagenesis of A553: <br> | |||
5'- CAT ATG GCG GCT GGT GTT AAG CAA CTG GCG GAT GAC CGC ACG CTG CTG ATG | |||
'''RNS''' GGG GTA AGT CAC GAC TTG CGC ACG CCG CTG ACG CGT -3' | |||
*'''NO295''': K-P+ control, mutagenesis of H557A (CAC to GCC)<br> | |||
5'- T GAC CGC ACG CTG CTG ATG GCG GGG GTA AGT '''GCC''' GAC TTG CGC ACG CCG CTG ACG CGT -3' | |||
*'''NO296''': fwd seq primer, starts at ~3500 in pCph8<br> | |||
5'- TCG TCA ACC TCA TTT TGC GCC AG -3'<br> | |||
*23 mer, Tm = 60.3 ºC | |||
*'''NO297''': fwd seq primer, start just upstream of Cph8 promoter<br> | |||
5' TCATGACATTAACCTATAAAAATAGGCGTATCACG | |||
*in map bp 1860-1894 | |||
*35mer, Tm 58.2°C | |||
==Useful links== | ==Useful links== | ||
*[http://openwetware.org/wiki/BE.109:Systems_engineering | *[http://openwetware.org/wiki/BE.109:Systems_engineering Original module] | ||
*[http://openwetware.org/wiki/BE.109:Protein_engineering/Assessing_beta-galactosidase | *[http://openwetware.org/wiki/BE.109:Protein_engineering/Assessing_beta-galactosidase b-gal assay] | ||
*[http://www.biocompare.com/Articles/ProductReview/342/Stratagene's-QuikChange-II-Site-Directed-Mutagenesis-Kit.html | *[http://www.biocompare.com/Articles/ProductReview/342/Stratagene's-QuikChange-II-Site-Directed-Mutagenesis-Kit.html SDM kit][http://openwetware.org/wiki/20.109%28S09%29:Site-directed_mutagenesis_%28Day2%29 SDM lab protocol] | ||
*[http://parts2.mit.edu/wiki/index.php/University_of_California_San_Francisco_2006 | *[http://parts2.mit.edu/wiki/index.php/University_of_California_San_Francisco_2006 controlled swimming][http://www.woodrow.org/teachers/bi/1998/people/omoto/day1.html chemotaxis lab protocol] | ||
*[http://www.biology.utah.edu/jorgensen/wayned/ape/ | *[http://www.biology.utah.edu/jorgensen/wayned/ape/ APE] | ||
*[http://www.biolog.com/PM_Services.html | *[http://www.biolog.com/PM_Services.html phenotype arrays] | ||
*[http://openwetware.org/wiki/QRT-PCR | *[http://openwetware.org/wiki/QRT-PCR q-PCR] | ||
*[http://www.neb.com/nebecomm/tech_reference/general_data/genetic_code.asp genetic code] | |||
*[http://www.genewiz.com/ Genewiz] | |||
==Useful Images and Files== | ==Useful Images and Files== | ||
| Line 27: | Line 64: | ||
As for the EnvZ mutants, here's the list we've compiled from the literature. Keep in mind that K+P- really means a shift in the balance of kinase and phosphatase activities and similarly for the K-P+ alleles. None of them is perfectly "clean" in eliminating one of the activities: | As for the EnvZ mutants, here's the list we've compiled from the literature. Keep in mind that K+P- really means a shift in the balance of kinase and phosphatase activities and similarly for the K-P+ alleles. None of them is perfectly "clean" in eliminating one of the activities: | ||
===K+P-=== | ===K+P-=== | ||
*V241G | *V241G '''= V555G in Cph8''' | ||
*G240E | *G240E '''= G554E in Cph8''' | ||
*S242D | *S242D '''= S556D in Cph8''' | ||
*P248Q | *P248Q | ||
*T247R | *T247R | ||
| Line 36: | Line 73: | ||
*L288P | *L288P | ||
===K-P+=== | ===K-P+=== | ||
*A239T | *A239T '''= A553 in Cph8''' | ||
*N343K | *N343K | ||
*F390L | *F390L | ||
*H243A | *H243A '''= H557A in Cph8''' | ||
NK note: relevant reference: | NK note: relevant reference: | ||
*Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. Hsing W, Russo FD, Bernd KK, Silhavy TJ. J Bacteriol. 1998 Sep;180(17):4538-46. [[PMID: 9721293]] | *Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. Hsing W, Russo FD, Bernd KK, Silhavy TJ. J Bacteriol. 1998 Sep;180(17):4538-46. [[PMID: 9721293]] | ||
*The role of the G2 box, a conserved motif in the histidine kinase superfamily, in modulating the function of EnvZ. Zhu Y, Inouye M. Mol Microbiol. 2002 Aug;45(3):653-63. [[PMID: 12139613]] | *The role of the G2 box, a conserved motif in the histidine kinase superfamily, in modulating the function of EnvZ. Zhu Y, Inouye M. Mol Microbiol. 2002 Aug;45(3):653-63. [[PMID: 12139613]] | ||
[[Image:UniqueSitesCph1-EnvZ.png|thumb|left| unique sited in Cph8, clone with Nde at 1546 and Mlu at 1630]] | |||
[[Image:RegionForRandomization.png|thumb|right| zoom-in seq of region for library]] | |||
[[Image:RandomizedKPlibrary.png]] | |||
===Primers=== | |||
'''NO281 = KP randomized template''' | |||
*5' CATATGGCGGCTGGTGTTAAGCAACTGGCGGATGACCGCACGCTGCTGATG RNS RNS RNS RNS SNW GACTTGCGCACGCCGCTGACGCGT<br> | |||
'''NO284 = P+ randomized template''' | |||
*5' CATATGGCGGCTGGTGTTAAGCAACTGGCGGATGACCGCACGCTGCTGATG RNS GGG GTA AGT SNW GACTTGCGCACGCCGCTGACGCGT<br> | |||
'''NO285 = K+ randomized template''' | |||
*5' CATATGGCGGCTGGTGTTAAGCAACTGGCGGATGACCGCACGCTGCTGATG GCG RNS RNS RNS CAC GACTTGCGCACGCCGCTGACGCGT<br> | |||
'''NO282 = KPLibrary_fwd_1535_1573''' | |||
*5' CAGAAGAATTG''CATATG''GCGGCTGGTGTTAAGCAACTGG | |||
**39 mer | |||
**Tm (all) = 66.3 ºC | |||
**Tm (landing, 28 mer) = 63.3 ºC | |||
'''NO283 = KPLibrary_rev_1643_1609''' | |||
*5' AGGCGAAT''ACGCGT''CAGCGGCGTGCGCAAGT | |||
**31 mer | |||
**Tm (all) = 72.1 ºC | |||
**Tm (landing, 23 mer) = 70.5 ºC<br> | |||
'''NO290''' =''' LibraryBuilder_rev''' | |||
**33mer | |||
**5' ATC CGC CAG TTG CTT AAC ACC AGC CGC CAT ATG | |||
**Tm = 67° | |||
** should hybridize to 5' end of K+ and P+ template oligos (NO284, NO285) to go 'round the horn for library | |||
'''NO279 = GFPfiller_Nde_fwd'''<br> | |||
5' CATTAGCATATGGGATCCTAAGAGGGCGAGGGCGATGCCACC | |||
** 42 mer | |||
**Tm (all) = 69.7 ºC | |||
**Tm (landing, 24 mer) = 67 ºC | |||
'''NO280 = GFPfiller_Mlu_rev'''<br> | |||
5' CATTAGTGCGCAGATATCTTACTTGTACAGCTCGTCCATGC<br> | |||
corrected: 5' CATTAGACGCGTGATATCTTACTTGTACAGCTCGTCCATGC | |||
**41 mer | |||
**Tm (all) = 64.6 ºC | |||
**Tm (landing, 23 mer) = 57 ºC<br> | |||
'''NO288= GFPfiller_Nde_rev<br>''' | |||
5'CATTAGCATATGGATATCTTACTTGTACAGCTCGTCCATGC | |||
**Tm (all) = 62.6 ºC | |||
**Tm (landing, 23 mer) = 57 ºC<br> | |||
'''NO293= sequencing pCph8 around K+ randomized sequences'''<br> | |||
5'CTC ATA GCC ACT TTC GGC AGC AAT | |||
**21 mer | |||
**Tm = 60° | |||
==Possible ways to epitope tag== | |||
[[Image:CloningEpitopeTags.png]] | |||
All tags at C-terminus, with intention to clone product using unique MfeI and XbaI in pCph8 | |||
===HA=== | |||
* 9-amino acid sequence (YPYDVPDYA) recognized by the anti-12CA5 (=anti-HA) | |||
* reverse translate at [http://slam.bs.jhmi.edu/gd/ |Gene Design] with E. coli codon bias | |||
* default settings gives sequence: TAC CCG TAC GAC GTT CCG GAC TAC GCT <br> | |||
'''caattg'''TGCAGCGTATCGTGGATAACCATAACGGGATGCTGGAGCTTGGCACCAGCGAGCGGGGCGGGCTTTCCATTCGCGCCTGGCTGCCAGTGCCGGTAACGCGGGCGCAGGGCATGACAAAAGAAGGG''TACCCGTACGACGTTCCGGACTACGCT''TAA'''tctaga | |||
====to PCR from DNA2.0 vector==== | |||
*NO286=HAfrag_fwd | |||
** 5' GTCGCTGAACAATTGTGCAGCGTATCGTGG | |||
** Tm = 61.0 ºC | |||
*NO287=HAfrag_rev | |||
** 5' GAACTCGATTGACGTCTAGATTAAGCGTAG | |||
** Tm = 58.1 ºC | |||
====to check insert of HA in pCph8==== | |||
* NO289 = Cph8+HAseqprimer_bp135_rev | |||
** 5' ATTACCGCCTTTGAGTGAGC | |||
===His6=== | |||
* 6 Histidine sequence from [http://www.emdbiosciences.com/docs/NDIS/71327-000.html pET-45b(+)] bp 4963-4980 (Novagen product) | |||
'''caattg'''TGCAGCGTATCGTGGATAACCATAACGGGATGCTGGAGCTTGGCACCAGCGAGCGGGGCGGGCTTTCCATTCGCGCCTGGCTGCCAGTGCCGGTAACGCGGGCGCAGGGCATGACAAAAGAAGGG''catcaccaccaccatcac''TAA'''tctaga''' | |||
Latest revision as of 09:02, 15 September 2012
Module for F'12
Mutagenesis of T541
Oligos for screen
- NO304: K+P- screen of T541, mutagenesis oligo:
5'--CTG GCG GAT GAC CGC ACG CTG CTG ATG GCG GGG GTA AGT CAC GAC TTG CGC NNY CCG CTG ACG CGT ATT CGC CTG GCG ACT GAG ATG ATG -3'
- NO305: complementary oligo that binds to 3' end of mutagenic oligo and builds plasmid around the horn:
5'- CAT CAT CTC AGT CGC CAG GCG AAT ACG CGT CAG -3'
Module for F'11
- Remade reporter strain NB462 and tested for induction--using antibiotics at: Amp25, Cam34, Kan10, and including Kan10 always.
- Remade library K+P- and K-P+. Used H557A as control for K-P+ screen
- Retested indicator media. Phenol red failed, but Z4 gave clear colonies in dark and red colonies in light (except where dense).
Students will try to identify K-P+ mutants that appear more red in light, i.e. lower units in light, then check to see if dynamic range improved or if all activity diminished
- Day 1: set up dark/light system in plates, liq culture
- Day 2: b-gal assay of cultures, set up photo
- Day 3: transform library and screen, recapitulate setup electronically
- Day 4: Journal club I, re-streak candidate, <teacher ONs in light and dark>
- Day 5: DNA for seq, and b-gal assay
- Day 6: Protein gel and blot, seq data, photo?, other assays
- Day 7: Probe blot, other assays
- Day 8: Journal club, data back
Oligos ordered for F'11 screen
- NO294: K-P+ library, mutagenesis of A553:
5'- CAT ATG GCG GCT GGT GTT AAG CAA CTG GCG GAT GAC CGC ACG CTG CTG ATG RNS GGG GTA AGT CAC GAC TTG CGC ACG CCG CTG ACG CGT -3'
- NO295: K-P+ control, mutagenesis of H557A (CAC to GCC)
5'- T GAC CGC ACG CTG CTG ATG GCG GGG GTA AGT GCC GAC TTG CGC ACG CCG CTG ACG CGT -3'
- NO296: fwd seq primer, starts at ~3500 in pCph8
5'- TCG TCA ACC TCA TTT TGC GCC AG -3'
- 23 mer, Tm = 60.3 ºC
- NO297: fwd seq primer, start just upstream of Cph8 promoter
5' TCATGACATTAACCTATAAAAATAGGCGTATCACG
- in map bp 1860-1894
- 35mer, Tm 58.2°C
Useful links
- Original module
- b-gal assay
- SDM kitSDM lab protocol
- controlled swimmingchemotaxis lab protocol
- APE
- phenotype arrays
- q-PCR
- genetic code
- Genewiz
Useful Images and Files




References
- Synthetic biology: engineering Escherichia coli to see light. Levskaya A, Chevalier AA, Tabor JJ, Simpson ZB, Lavery LA, Levy M, Davidson EA, Scouras A, Ellington AD, Marcotte EM, Voigt CA.
Nature. 2005 Nov 24;438(7067):441-2. PMID: 16306980 and pdf
- Engineered single- and multi-cell chemotaxis pathways in E. coli. Goldberg SD, Derr P, DeGrado WF, Goulian M. Mol Syst Biol. 2009;5:283. Epub 2009 Jun 16. PMID: 19536206 and pdf
- Specificity in two-component signal transduction pathways. Laub MT, Goulian M. Annu Rev Genet. 2007;41:121-45. Review. PMID: 18076326 and pdf
- Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. Hsing W, Russo FD, Bernd KK, Silhavy TJ. J Bacteriol. 1998 Sep;180(17):4538-46. PMID: 9721293 and pdf
- Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Siryaporn A, Goulian M. Mol Microbiol. 2008 Oct;70(2):494-506. Epub 2008 Aug 29. PMID: 18761686 and pdf
- Design and signaling mechanism of light-regulated histidine kinases. Möglich A, Ayers RA, Moffat K. J Mol Biol. 2009 Feb 6;385(5):1433-44. Epub 2008 Dec 14. PMID: 19109976 and pdf
- EnvZ-OmpR interaction and osmoregulation in Escherichia coli. Cai SJ, Inouye M. J Biol Chem. 2002 Jul 5;277(27):24155-61. Epub 2002 Apr 24. PMID: 11973328 and pdf
K-P+ and K+P- mutations
06.16.09 email from Mike: As for the EnvZ mutants, here's the list we've compiled from the literature. Keep in mind that K+P- really means a shift in the balance of kinase and phosphatase activities and similarly for the K-P+ alleles. None of them is perfectly "clean" in eliminating one of the activities:
K+P-
- V241G = V555G in Cph8
- G240E = G554E in Cph8
- S242D = S556D in Cph8
- P248Q
- T247R
- Q283P
- Y287D
- L288P
K-P+
- A239T = A553 in Cph8
- N343K
- F390L
- H243A = H557A in Cph8
NK note: relevant reference:
- Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. Hsing W, Russo FD, Bernd KK, Silhavy TJ. J Bacteriol. 1998 Sep;180(17):4538-46. PMID: 9721293
- The role of the G2 box, a conserved motif in the histidine kinase superfamily, in modulating the function of EnvZ. Zhu Y, Inouye M. Mol Microbiol. 2002 Aug;45(3):653-63. PMID: 12139613


Primers
NO281 = KP randomized template
- 5' CATATGGCGGCTGGTGTTAAGCAACTGGCGGATGACCGCACGCTGCTGATG RNS RNS RNS RNS SNW GACTTGCGCACGCCGCTGACGCGT
NO284 = P+ randomized template
- 5' CATATGGCGGCTGGTGTTAAGCAACTGGCGGATGACCGCACGCTGCTGATG RNS GGG GTA AGT SNW GACTTGCGCACGCCGCTGACGCGT
NO285 = K+ randomized template
- 5' CATATGGCGGCTGGTGTTAAGCAACTGGCGGATGACCGCACGCTGCTGATG GCG RNS RNS RNS CAC GACTTGCGCACGCCGCTGACGCGT
NO282 = KPLibrary_fwd_1535_1573
- 5' CAGAAGAATTGCATATGGCGGCTGGTGTTAAGCAACTGG
- 39 mer
- Tm (all) = 66.3 ºC
- Tm (landing, 28 mer) = 63.3 ºC
NO283 = KPLibrary_rev_1643_1609
- 5' AGGCGAATACGCGTCAGCGGCGTGCGCAAGT
- 31 mer
- Tm (all) = 72.1 ºC
- Tm (landing, 23 mer) = 70.5 ºC
NO290 = LibraryBuilder_rev
- 33mer
- 5' ATC CGC CAG TTG CTT AAC ACC AGC CGC CAT ATG
- Tm = 67°
- should hybridize to 5' end of K+ and P+ template oligos (NO284, NO285) to go 'round the horn for library
NO279 = GFPfiller_Nde_fwd
5' CATTAGCATATGGGATCCTAAGAGGGCGAGGGCGATGCCACC
- 42 mer
- Tm (all) = 69.7 ºC
- Tm (landing, 24 mer) = 67 ºC
NO280 = GFPfiller_Mlu_rev
5' CATTAGTGCGCAGATATCTTACTTGTACAGCTCGTCCATGC
corrected: 5' CATTAGACGCGTGATATCTTACTTGTACAGCTCGTCCATGC
- 41 mer
- Tm (all) = 64.6 ºC
- Tm (landing, 23 mer) = 57 ºC
NO288= GFPfiller_Nde_rev
5'CATTAGCATATGGATATCTTACTTGTACAGCTCGTCCATGC
- Tm (all) = 62.6 ºC
- Tm (landing, 23 mer) = 57 ºC
NO293= sequencing pCph8 around K+ randomized sequences
5'CTC ATA GCC ACT TTC GGC AGC AAT
- 21 mer
- Tm = 60°
Possible ways to epitope tag
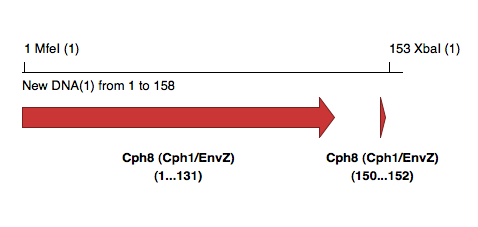 All tags at C-terminus, with intention to clone product using unique MfeI and XbaI in pCph8
All tags at C-terminus, with intention to clone product using unique MfeI and XbaI in pCph8
HA
- 9-amino acid sequence (YPYDVPDYA) recognized by the anti-12CA5 (=anti-HA)
- reverse translate at |Gene Design with E. coli codon bias
- default settings gives sequence: TAC CCG TAC GAC GTT CCG GAC TAC GCT
caattgTGCAGCGTATCGTGGATAACCATAACGGGATGCTGGAGCTTGGCACCAGCGAGCGGGGCGGGCTTTCCATTCGCGCCTGGCTGCCAGTGCCGGTAACGCGGGCGCAGGGCATGACAAAAGAAGGGTACCCGTACGACGTTCCGGACTACGCTTAAtctaga
to PCR from DNA2.0 vector
- NO286=HAfrag_fwd
- 5' GTCGCTGAACAATTGTGCAGCGTATCGTGG
- Tm = 61.0 ºC
- NO287=HAfrag_rev
- 5' GAACTCGATTGACGTCTAGATTAAGCGTAG
- Tm = 58.1 ºC
to check insert of HA in pCph8
- NO289 = Cph8+HAseqprimer_bp135_rev
- 5' ATTACCGCCTTTGAGTGAGC
His6
- 6 Histidine sequence from pET-45b(+) bp 4963-4980 (Novagen product)
caattgTGCAGCGTATCGTGGATAACCATAACGGGATGCTGGAGCTTGGCACCAGCGAGCGGGGCGGGCTTTCCATTCGCGCCTGGCTGCCAGTGCCGGTAACGCGGGCGCAGGGCATGACAAAAGAAGGGcatcaccaccaccatcacTAAtctaga
