BE.109:DNA engineering: Difference between revisions
(Undo revision 157507 by Special:Contributions/Bevin (User talk:Bevin)) |
|||
| (46 intermediate revisions by 3 users not shown) | |||
| Line 12: | Line 12: | ||
In this experimental module you will modify the gene for ''EGFP'' (Enhanced Green Fluorescent Protein) to truncate the protein it encodes. Cells expressing the full-length protein glow green when exposed to light of the appropriate wavelength. You will be designing and then creating an expression vector to delete the first 32 amino acids of EGFP. Cells transfected with your expression vector should not glow green, a prediction you will test. You will also test whether this N-terminally truncated EGFP can recombine with a C-terminally truncated version to regenerate full length EGFP in vivo. Finally, you will have the opportunity to suggest changes to the experimental protocol that will increase the frequency of green cells in which there has been an inter-plasmid recombination event. We will then choose a few variables to test on the final day of the experiment. | In this experimental module you will modify the gene for ''EGFP'' (Enhanced Green Fluorescent Protein) to truncate the protein it encodes. Cells expressing the full-length protein glow green when exposed to light of the appropriate wavelength. You will be designing and then creating an expression vector to delete the first 32 amino acids of EGFP. Cells transfected with your expression vector should not glow green, a prediction you will test. You will also test whether this N-terminally truncated EGFP can recombine with a C-terminally truncated version to regenerate full length EGFP in vivo. Finally, you will have the opportunity to suggest changes to the experimental protocol that will increase the frequency of green cells in which there has been an inter-plasmid recombination event. We will then choose a few variables to test on the final day of the experiment. | ||
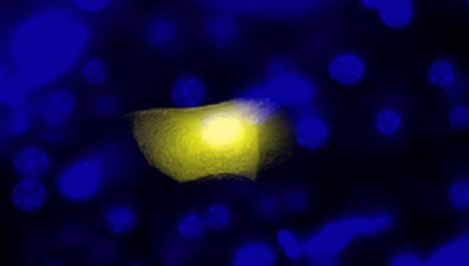
[[Image:Be109recombomouse.jpg|thumb|left|500px|'''Recombocell | [[Image:Be109recombomouse.jpg|thumb|left|500px|'''Recombocell image from Dominika Wiktor of the Engelward Lab''']] | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
A schematic overview of | [[Image:Experimental Overview.jpg|thumb|left|500px|'''A schematic overview of the module.''']] | ||
[[Image:Experimental | <br style="clear:both" /> | ||
[[Image:Experimental Overview1.jpg|thumb|left|500px|'''Timetable of the module.''']] | |||
<br style="clear:both" /> | <br style="clear:both" /> | ||
| Line 38: | Line 40: | ||
'''[[BE.109: | '''[[BE.109:DNA engineering/Lab report | Module 1 lab report schedule and guidelines ]]''' | ||
==DNA engineering web links== | ==DNA engineering web links== | ||
| Line 44: | Line 46: | ||
Engelward lab resources: https://web.mit.edu/bevin/www/UltiMouse/ | Engelward lab resources: https://web.mit.edu/bevin/www/UltiMouse/ | ||
pCX-EGFP plasmid map: https://web.mit.edu/bevin/www/UltiMouse/pCX-EGFP.pdf [[Image:Macintosh_HD-Users-nkuldell-Desktop-pCX-EGFP.doc|pCX-EGFP | pCX-EGFP plasmid map: https://web.mit.edu/bevin/www/UltiMouse/pCX-EGFP.pdf [[Image:Macintosh_HD-Users-nkuldell-Desktop-pCX-EGFP.doc|pCX-EGFP]] | ||
ORF finder: http://www.ncbi.nlm.nih.gov/gorf/gorf.html | ORF finder: http://www.ncbi.nlm.nih.gov/gorf/gorf.html | ||
| Line 58: | Line 60: | ||
'''Note:''' PDF reprints are provided below within the context of [http://www.copyright.gov/fls/fl102.html fair use]. Please obtain copies from the publisher if appropriate. | '''Note:''' PDF reprints are provided below within the context of [http://www.copyright.gov/fls/fl102.html fair use]. Please obtain copies from the publisher if appropriate. | ||
#'''Pathways for mitotic homologous recombination in mammalian cells'''<br>''Mutation Research'' 27 November 2003 | #'''Pathways for mitotic homologous recombination in mammalian cells'''<br>''Mutation Research'' 27 November 2003<br> Thomas Helleday<br> [http://dx.doi.org/10.1016/j.mrfmmm.2003.08.013 URL] | ||
#'''Homologous recombination as a mechanism of carcinogenesis'''<br>'' Biochim Biophys Acta'' 21 March 2001<br> Bishop AJ and Schiestl RH<br> [http://dx.doi.org/10.1016/S0304-419X(01)00018-X URL] | |||
#'''Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death'''<br>'' EMBO J'' 15 January 1998<br> E Sonoda, M S Sasaki, J M Buerstedde, O Bezzubova, A Shinohara, H Ogawa, M Takata, Y Yamaguchi-Iwai, and S Takeda<br> [http://www.pubmedcentral.gov/picrender.fcgi?artid=1170409&blobtype=pdf PDF reprint] | |||
Latest revision as of 10:32, 10 October 2007
Module 1
Instructors: Bevin Engelward and Natalie Kuldell
TA: Yoon Sung Nam
In this experimental module you will modify the gene for EGFP (Enhanced Green Fluorescent Protein) to truncate the protein it encodes. Cells expressing the full-length protein glow green when exposed to light of the appropriate wavelength. You will be designing and then creating an expression vector to delete the first 32 amino acids of EGFP. Cells transfected with your expression vector should not glow green, a prediction you will test. You will also test whether this N-terminally truncated EGFP can recombine with a C-terminally truncated version to regenerate full length EGFP in vivo. Finally, you will have the opportunity to suggest changes to the experimental protocol that will increase the frequency of green cells in which there has been an inter-plasmid recombination event. We will then choose a few variables to test on the final day of the experiment.


Lab handouts
Day 1: DNA engineering using PCR (you will also need weblinks, below)
Day 3: Agarose gel electrophoresis
Day 4: DNA ligation and bacterial transformation
Day 5: Examine candidate clones
Day 6: Restriction map and tissue culture
Module 1 lab report schedule and guidelines
DNA engineering web links
Engelward lab resources: https://web.mit.edu/bevin/www/UltiMouse/
pCX-EGFP plasmid map: https://web.mit.edu/bevin/www/UltiMouse/pCX-EGFP.pdf File:Macintosh HD-Users-nkuldell-Desktop-pCX-EGFP.doc
ORF finder: http://www.ncbi.nlm.nih.gov/gorf/gorf.html
NCBI: http://www.ncbi.nlm.nih.gov/
Cybergene: http://www.cybergene.se/primer.html
New England Biolabs: http://www.neb.com/nebecomm/default.asp
References
Note: PDF reprints are provided below within the context of fair use. Please obtain copies from the publisher if appropriate.
- Pathways for mitotic homologous recombination in mammalian cells
Mutation Research 27 November 2003
Thomas Helleday
URL - Homologous recombination as a mechanism of carcinogenesis
Biochim Biophys Acta 21 March 2001
Bishop AJ and Schiestl RH
URL - Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death
EMBO J 15 January 1998
E Sonoda, M S Sasaki, J M Buerstedde, O Bezzubova, A Shinohara, H Ogawa, M Takata, Y Yamaguchi-Iwai, and S Takeda
PDF reprint
