BIO254:Exam2002: Difference between revisions
No edit summary |
|||
| Line 7: | Line 7: | ||
Describe three major similarities and three major differences (biology and/or mechanism) of Rac (a Rho family small GTPase) and Transducin (a trimeric G protein) (The fact that they are both GTPases is a given now.) | Describe three major similarities and three major differences (biology and/or mechanism) of Rac (a Rho family small GTPase) and Transducin (a trimeric G protein) (The fact that they are both GTPases is a given now.) | ||
Differences: | <b>Differences: | ||
Rac is a small GTPase and is monomeric | Rac is a small GTPase and is monomeric | ||
| Line 25: | Line 25: | ||
They both get activated by the release of GDP and binding to GTP. | They both get activated by the release of GDP and binding to GTP. | ||
They both get deactivated by the hydrolysis or release of GTP and binding of GDP | They both get deactivated by the hydrolysis or release of GTP and binding of GDP | ||
</b> | |||
==II. (24 pts)== | ==II. (24 pts)== | ||
(a) While searching the human genome you come across a putative receptor for axon guidance based on its conserved motifs with other known axon guidance receptors. You are happy to find out that this receptor has unstudied homologues in other organisms including worm, fly, zebra fish, and mouse. Give two reasons why you should be happy about that (4 pts). | (a) While searching the human genome you come across a putative receptor for axon guidance based on its conserved motifs with other known axon guidance receptors. You are happy to find out that this receptor has unstudied homologues in other organisms including worm, fly, zebra fish, and mouse. Give two reasons why you should be happy about that (4 pts). | ||
<b> | |||
1. The existence of homologues in so many model organisms makes available for experimentation, all the different advantages of each system and the genetic tools that come with them. You have a whole range of possibilities in studying the receptor in different models and comparing their functions. | 1. The existence of homologues in so many model organisms makes available for experimentation, all the different advantages of each system and the genetic tools that come with them. You have a whole range of possibilities in studying the receptor in different models and comparing their functions. | ||
| Line 34: | Line 34: | ||
2. The genetic conservation across species also means that this is most likely a very important gene, that probably also exists in homologous form in humans | 2. The genetic conservation across species also means that this is most likely a very important gene, that probably also exists in homologous form in humans | ||
</b> | |||
(b) You decide to make a loss-of-function mutant of this putative receptor in fly. How will you determine where to look in your mutant animal for phenotypes? (2 pts) Give a reason why you might not see a phenotype even if you have “knocked out” the gene activity completely (2 pts). | (b) You decide to make a loss-of-function mutant of this putative receptor in fly. How will you determine where to look in your mutant animal for phenotypes? (2 pts) Give a reason why you might not see a phenotype even if you have “knocked out” the gene activity completely (2 pts). | ||
| Line 88: | Line 88: | ||
1) According to the picture above, what step of the photoreceptor response is affected by X mutant? (4 pts) | 1) According to the picture above, what step of the photoreceptor response is affected by X mutant? (4 pts) | ||
The increased amplitude and prolonged response in the mutant suggests that there is a defect in the deactivation step of the phototransduction cascade. | <b>The increased amplitude and prolonged response in the mutant suggests that there is a defect in the deactivation step of the phototransduction cascade. </b> | ||
2) What protein could gene X encode? Give three candidates discussed in lectures. Explain how mutations in these three candidate genes could result in the phenotypes seen in the graph. (12 pts) | 2) What protein could gene X encode? Give three candidates discussed in lectures. Explain how mutations in these three candidate genes could result in the phenotypes seen in the graph. (12 pts) | ||
| Line 129: | Line 129: | ||
1) What is the difference between voltage-dependent closure of a channel and inactivation of a channel? (3 pts) | 1) What is the difference between voltage-dependent closure of a channel and inactivation of a channel? (3 pts) | ||
The voltage-dependent closure of a channel occurs at an activation gate when there is a change in voltage. Channel inactivation occurs at the inactivation gate when the voltage is maintained, for example, when the membrane remains depolarized. | <b>The voltage-dependent closure of a channel occurs at an activation gate when there is a change in voltage. Channel inactivation occurs at the inactivation gate when the voltage is maintained, for example, when the membrane remains depolarized. </b> | ||
2) Describe one example in which inactivation independent of voltage-dependent closure is important. (3 pts) | 2) Describe one example in which inactivation independent of voltage-dependent closure is important. (3 pts) | ||
Inactivation of Na channels is important to limit the duration of an action potential. Upon depolarization, Na channels rapidly open, creating the rising phase of the action potential, but soon after the inactivation gates will gradually close limiting further Na influx. | <b>Inactivation of Na channels is important to limit the duration of an action potential. Upon depolarization, Na channels rapidly open, creating the rising phase of the action potential, but soon after the inactivation gates will gradually close limiting further Na influx. </b> | ||
3) Describe one mechanism by which inactivation is achieved. (2 pts) | 3) Describe one mechanism by which inactivation is achieved. (2 pts) | ||
In the Na channel, inactivation occurs though a ball and chain model, in which the "ball" blocks the opening of the channel when the inactivation gate is closed. | <b>In the Na channel, inactivation occurs though a ball and chain model, in which the "ball" blocks the opening of the channel when the inactivation gate is closed.</b> | ||
Revision as of 04:36, 5 December 2006
Bio154 Sample Exam 2002-Key
NOTE: Answers are provided by your classmates. Some questions may have multiple correct answers; as long as yours is reasonable, logic and consistent, you can get full credit. Some questions may be challenging but don’t panic; grading will be on a curve. Good luck.
I. (12 pts)
Describe three major similarities and three major differences (biology and/or mechanism) of Rac (a Rho family small GTPase) and Transducin (a trimeric G protein) (The fact that they are both GTPases is a given now.)
Differences:
Rac is a small GTPase and is monomeric Whereas Transducin is heterotrimeric
Rac (small G proteins) can act as a Biological timer. This is because they can convert from the GTP bound form to the GDP bound form almost cyclically. Transducin (heterotrimeric G-proteins) on the other hand, require GDP dissociation factors to dissociate and go back to the GTP bound form. These proteins are called GEFs guanisine nucleotide exchange factors.
Rac plays a role in actin polymerization at the growth cone during development or reorganization. It responds to chemoattractants present in this region. Transducin plays a role in vision consitently throughout life via the conformational change in rhodopsin (mediated by photon absorption) Transducin is membrane associated and works with GPRCs to function in vision
Similarities:
They are both involved in cell signaling and signal transduction They both have alpha, beta and gamma subunits They both get activated by the release of GDP and binding to GTP. They both get deactivated by the hydrolysis or release of GTP and binding of GDP
II. (24 pts)
(a) While searching the human genome you come across a putative receptor for axon guidance based on its conserved motifs with other known axon guidance receptors. You are happy to find out that this receptor has unstudied homologues in other organisms including worm, fly, zebra fish, and mouse. Give two reasons why you should be happy about that (4 pts).
1. The existence of homologues in so many model organisms makes available for experimentation, all the different advantages of each system and the genetic tools that come with them. You have a whole range of possibilities in studying the receptor in different models and comparing their functions.
2. The genetic conservation across species also means that this is most likely a very important gene, that probably also exists in homologous form in humans
(b) You decide to make a loss-of-function mutant of this putative receptor in fly. How will you determine where to look in your mutant animal for phenotypes? (2 pts) Give a reason why you might not see a phenotype even if you have “knocked out” the gene activity completely (2 pts).
(c) Wow! Your mutant has a striking phenotype. You notice that in a subpopulation of neurons your mutant axons fail to make a critical turn at a specific point (neuron A). You are not sure if your newly discovered receptor acts as a receptor for an attractive or repulsive cue. Draw two models postulating an attractive or repulsive cue. (6 pts)
Wild-type Mutant
(d) You also notice that in wild-type animals another subpopulation of neurons never make this turn (neuron B). Propose an experiment to show that your receptor is sufficient for axon turning in this system. (6 pts)
Wild-type Wild-type
(e) You are now interested in what is downstream of your receptor. Propose two general experimental techniques that may help you answer this question. (4 pts)
III. (24 pts)
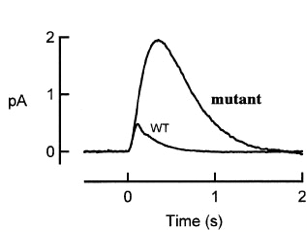
An interesting mutant of gene X has been found in the course of photoreceptor study. The figure below shows the population mean single photon responses of rods from wild type and from mutant X.
1) According to the picture above, what step of the photoreceptor response is affected by X mutant? (4 pts)
The increased amplitude and prolonged response in the mutant suggests that there is a defect in the deactivation step of the phototransduction cascade.
2) What protein could gene X encode? Give three candidates discussed in lectures. Explain how mutations in these three candidate genes could result in the phenotypes seen in the graph. (12 pts)
3) Come up with two further experiments to distinguish your 3 hypotheses in (2). (8 pts)
IV. (24 pts)
To investigate the mechanisms of retinocollicular (mammalian equivalent of retinotectal) projection specificity, O’Leary and colleagues generated knock-out mice for both Ephrin B2 and B3 receptors (EphB2 & EphB3). To study the phenotype, they inject an axon tracer DiI into a very small and identical region of the retina in both WT and double knockout mice, and then analyze where these RGC axons terminate in the superior colliculus by following DiI labeled axons. The figure shows the DiI labeled RGC axon termination in the superior colliculus for WT and double knockout mice (L: lateral; M: medial; P: posterior; Anterior border is indicated by the arrowheads).
...............WT....................EphB2-/-;EphB3-/-.....
1) Judging from the location of the termination in the superior colliculus in WT, which part of the retina do you think they inject their DiI? (4 pts)
2) In class, we mentioned that EphBs are expressed in RGC in a high ventral-low dorsal gradient; and Ephrin Bs are expressed in superior colliculus in a high medial-low lateral gradient. How can you explain the double knock-out phenotype? (4 pts)
3) If EphB2 and EphB3 are the only receptors for D-V patterning, you would imagine that without these receptors, axons will randomly distribute along the D-V axis. However you quickly notice that the ectopic terminal zones (eTZ) always project lateral to the correct terminal zone (TZ) instead diffusely in both directions (see Figure). Propose a simple model that explains this effect. (4 pts)
4) You may also have noticed that in EphB mutants axons in eTZ largely concentrate on one spot instead of being diffusive. Propose an explanation for this phenomenon and describe a simple experiment to test your hypothesis. (6 pts).
5) It turns out that Ephrin Bs are also expressed in RGC axons in a gradient fashion along D-V axis, and EphB are also expressed in superior colliculus (target) along a M-L axis! Moreover, since the “ligands” for EphBs, the Ephrin B, are transmembrane proteins so they can sometimes act as receptors for “reverse signaling”. So the phenotypes seen in the Figure is not necessarily caused by loss of EphBs in the axons; the loss of EphB in the target could also contribute. Describe one experiment that could distinguish whether EphB is needed in RGC axon or in the target for the correct establishment of topographic map along the D-V axis. (6 pts)
V. (16 pts)
1) What is the difference between voltage-dependent closure of a channel and inactivation of a channel? (3 pts)
The voltage-dependent closure of a channel occurs at an activation gate when there is a change in voltage. Channel inactivation occurs at the inactivation gate when the voltage is maintained, for example, when the membrane remains depolarized.
2) Describe one example in which inactivation independent of voltage-dependent closure is important. (3 pts)
Inactivation of Na channels is important to limit the duration of an action potential. Upon depolarization, Na channels rapidly open, creating the rising phase of the action potential, but soon after the inactivation gates will gradually close limiting further Na influx.
3) Describe one mechanism by which inactivation is achieved. (2 pts)
In the Na channel, inactivation occurs though a ball and chain model, in which the "ball" blocks the opening of the channel when the inactivation gate is closed.
4) Type-A potassium channels are a group of voltage-gated K channels that rapidly inactivate at membrane potentials at which the classic Hodgkin-Huxley voltage dependent potassium channel stays active.
In the voltage-clamp experiment shown below, a molluscan neuron containing classic Hodgkin-Huxley K channels and type-A potassium channels was depolarized to 5 mV from a holding potential of either -40 mV or -80 mV. The resulting current is quite different: one (from -40mV) consists of only IK (the classic Hodgkin-Huxley channel), the other (from –80mV) consists of IK and IA (the current from the type A channel). One could thus deduce the IA current from subtraction (dotted line).

(a) What does this experiment reveal about the properties of the type-A channel? (4 pts)
(b) Type-A potassium channels prevent neurons from firing repetitively at high frequencies but enable them to fire repetitively at low frequencies. How do the properties of the type-A channel enable it to regulate the spacing of action potentials? (4 pts)
Recent updates to the site:
- N
- This edit created a new page (also see list of new pages)
- m
- This is a minor edit
- b
- This edit was performed by a bot
- (±123)
- The page size changed by this number of bytes
17 April 2024
|
|
15:34 | BioMicroCenter:Element Sequencing 3 changes history +295 [Challee (3×)] | |||
|
|
15:34 (cur | prev) +195 Challee talk contribs | ||||
|
|
14:22 (cur | prev) +100 Challee talk contribs | ||||
|
|
14:07 (cur | prev) 0 Challee talk contribs | ||||
| 13:10 | BioMicroCenter:SingleCell diffhist +30 Noelani Kamelamela talk contribs (→10X CHROMIUM X) | ||||
| 12:43 | BioMicroCenter diffhist −15 Noelani Kamelamela talk contribs | ||||
16 April 2024
|
|
N 19:59 | Nanoimprint Lithography (NIL) - Carter Paul 10 changes history +7,205 [CarterPaul (10×)] | |||
|
|
19:59 (cur | prev) +769 CarterPaul talk contribs (→Thermal NIL Process) | ||||
|
|
19:53 (cur | prev) 0 CarterPaul talk contribs (→Thermal NIL Process) | ||||
|
|
19:53 (cur | prev) 0 CarterPaul talk contribs (→Thermal NIL Process) | ||||
|
|
19:52 (cur | prev) +1 CarterPaul talk contribs (→Thermal NIL Process) | ||||
|
|
19:50 (cur | prev) +202 CarterPaul talk contribs (→Thermal NIL Process) | ||||
|
|
19:17 (cur | prev) −20 CarterPaul talk contribs (→References) | ||||
|
|
19:17 (cur | prev) −1 CarterPaul talk contribs | ||||
|
|
19:11 (cur | prev) +4,278 CarterPaul talk contribs | ||||
|
|
18:53 (cur | prev) +1,891 CarterPaul talk contribs | ||||
| N |
|
18:42 (cur | prev) +85 CarterPaul talk contribs (Created page with "{{Template:CHEM-ENG590E}} =Motivation= =Introduction to NIL= =Thermal NIL Process=") | |||
| 19:40 | Upload log CarterPaul talk contribs uploaded File:NIL1.png | ||||
| N 18:40 | 3D Cell Culture - McLean Taggart, Emma Villares, Maximillian Marek, Scott LeBlanc, Adam Lyons and Jacob Belden diffhist +24,060 CarterPaul talk contribs (Created page with "{{Template:CHEM-ENG590E}} ==Introduction== While most microfluidic devices incorporate a 2D cell culture design, in which a single layer of cells is grown on the bottom of a device, these systems suffer from poor <i>in vivo</i> mimicry, as, in the human body, most cells grow in all directions.<sup>https://doi.org/10.5114/aoms.2016.63743 1</sup> To address this limitation, 3D cell culture devices have been developed - in w...") | ||||
|
|
18:38 | CHEM-ENG590E:Wiki Textbook 2 changes history +63 [CarterPaul (2×)] | |||
|
|
18:38 (cur | prev) +50 CarterPaul talk contribs (→Chapter 1 - Microfabrication) | ||||
|
|
18:37 (cur | prev) +13 CarterPaul talk contribs | ||||
| 18:36 | 3D Cell Culture - McLean Taggart, Emma Villares, Maximillian Marek, Scott LeBlanc, and Adam Lyons diffhist +5,343 CarterPaul talk contribs (Added a Technique and applications section) | ||||
|
|
10:20 | Yarn Microfluidics - Roger Dirth 12 changes history +442 [Rcostello (12×)] | |||
|
|
10:20 (cur | prev) +41 Rcostello talk contribs (→Applications) | ||||
|
|
10:19 (cur | prev) +36 Rcostello talk contribs (→Applications) | ||||
|
|
10:18 (cur | prev) +36 Rcostello talk contribs (→Introduction) | ||||
|
|
10:17 (cur | prev) +38 Rcostello talk contribs (→Fabrication) | ||||
|
|
10:17 (cur | prev) +38 Rcostello talk contribs (→Washburn Equation) | ||||
|
|
10:16 (cur | prev) +38 Rcostello talk contribs (→Wicking Rate) | ||||
|
|
10:16 (cur | prev) +37 Rcostello talk contribs (→Introduction) | ||||
|
|
10:15 (cur | prev) +36 Rcostello talk contribs (→Wicking Rate) | ||||
|
|
10:14 (cur | prev) +36 Rcostello talk contribs (→Fabrication) | ||||
|
|
10:14 (cur | prev) +34 Rcostello talk contribs (→Applications) | ||||
|
|
10:14 (cur | prev) +36 Rcostello talk contribs (→Introduction) | ||||
|
|
10:13 (cur | prev) +36 Rcostello talk contribs (→Introduction) | ||||
|
|
08:18 | 3D Printed Microfluidic Robots - Helen Hua 2 changes history +6 [Michele Caggioni (2×)] | |||
|
|
08:18 (cur | prev) +22 Michele Caggioni talk contribs (→Actuation) | ||||
|
|
08:18 (cur | prev) −16 Michele Caggioni talk contribs (→Actuation) | ||||
| 08:11 | 3D Printing Overview diffhist +422 Michele Caggioni talk contribs | ||||
15 April 2024
|
|
23:43 | User:Yanbin Huang 2 changes history +170 [Yanbin Huang (2×)] | |||
|
|
23:43 (cur | prev) 0 Yanbin Huang talk contribs (→Granted Patents) | ||||
|
|
23:43 (cur | prev) +170 Yanbin Huang talk contribs (→Granted Patents) | ||||
|
|
22:11 | The paper that launched microfluidics - Xi Ning 11 changes history +4,793 [Xning098 (11×)] | |||
|
|
22:11 (cur | prev) −6 Xning098 talk contribs (→Summary) | ||||
|
|
22:07 (cur | prev) −12 Xning098 talk contribs (→Synthesis) | ||||
|
|
22:06 (cur | prev) 0 Xning098 talk contribs | ||||
|
|
22:06 (cur | prev) +1 Xning098 talk contribs | ||||
|
|
22:05 (cur | prev) 0 Xning098 talk contribs | ||||
|
|
22:03 (cur | prev) +630 Xning098 talk contribs | ||||
|
|
22:01 (cur | prev) +3,189 Xning098 talk contribs | ||||
|
|
21:44 (cur | prev) +688 Xning098 talk contribs (→Separation and quantification) | ||||
|
|
21:33 (cur | prev) +306 Xning098 talk contribs | ||||
|
|
21:29 (cur | prev) −2 Xning098 talk contribs (→Electrokinetic effect) | ||||
|
|
21:28 (cur | prev) −1 Xning098 talk contribs (→Separation and quantification) | ||||
|
|
21:45 | (Upload log) [Xning098 (2×)] | |||
|
|
21:45 Xning098 talk contribs uploaded File:Figure 4 Tdesign.png | ||||
|
|
21:30 Xning098 talk contribs uploaded File:Figure 3 Set-up3.png | ||||