BISC110/F11: Lab 8
Part 1 Photosynthesis 1: Spectrophometry and Chlorophyll in Photosynthesis; Creating a Standard Curve--Part 2: Taster Data Analysis & Science Writing Workshop
We are ending our investigation of the PTC taster gene and beginning a new investigation of photosynthesis. Your lab today is divided into two distinct parts. There will be a wet lab component where you will use the peak absorbance of the main photosynthetic pigment of plants, chlorophyll a, to determine the concentration of an unknown chlorophyll-like compound. You will learn to use an important lab instrument, the spectrophotometer, to measure the absorbance spectrum of this pigmented compound and to find the peak absorbance. You will use this peak absorbance to measure absorbance of an unknown and to determine its concentration. To accomplish this goal you will need to create a standard curve from measuring the absorbance a series of dilutions of known concentration of this pigmented compound. This experiment is based on the relationship between concentration and absorbance known as the Beer-Lambert Law.
In the second and third lab in this photosynthesis unit, we will examine various factors that influence photosynthetic electron transport rates. When scientists try to understand complex biological processes through lab experimentation, they often try to parse out an essential part of a complex process as a way of understanding that part and its relationship in a broader context. Sometimes scientists perform indirect measurements using indicators. In this series your goal is to understand some of the factors that drive the rate of photosynthesis by measuring indirectly one part of the energy transfer process.
The second part of Lab today will be a collaborative look at the draft figures created from the preliminary course taster data and a science writing workshop that focuses on the Introduction and Discussion sections of the primary research report that you will write about your PTC taster gene investigation.
Introduction
PHOTOSYNTHETIC PIGMENTS Chlorophyll a and b impart the green color that one associates with plant leaves. Carotenoids, which are yellow pigments, are also present in leaves but are usually masked by the chlorophylls. It is only in the fall when the chlorophylls are degraded faster than the carotenoids that the yellow color becomes visible to us. The chlorophyll and carotenoid contents of plants can vary markedly with its age, or depend on environmental factors such as light intensity or quality during growth. The pale green appearance of a willow tree in early spring is markedly different from its olive-green of late summer. The intense dark green of "shade adapted" plants differs from the lacy green colors one sees at the top of a forest canopy. In today’s lab we will measure the relative concentrations of photosynthetic pigments in the leaves of one dark green plant, spinach.
Carotenoids and chlorophylls are found in the chloroplasts and are associated with the thylakoids, the internal membrane network of these organelles. It is now established that all chlorophylls are organized as discrete chlorophyll-protein complexes within the lipid matrix of the photosynthetic membrane.
The majority of chlorophyll a molecules (and all chlorophyll b and carotenoid molecules) function as antenna pigments. In combination with proteins, they form the light-harvesting complexes, which absorb and funnel light energy to the reaction center chlorophylls, thereby allowing the plant to utilize a broad spectrum of wavelengths for photosynthesis. Some of the chlorophyll a molecules serve specialized functions in the reaction centers of photosystems I and II, where the light energy is used to drive the reduction of components of the electron transport chain.
Leaves contain usually 7 different carotenoids (neoxanthin, violaxanthin, lutein, zeaxanthin, antheraxanthin, β-carotene and α-carotene; see Fig.1) that function in light harvesting. All carotenoids are derived from a skeleton of 40 carbon atoms, linked with alternating unsaturated bonds. The terminal carbons are arranged in rings. Carotenoids such as lutein that contain oxygen are termed xanthophylls. As mentioned before these carotenoids appear yellow or orange in color, because they absorb light in the blue part of the visible light spectrum. One can determine the characteristic patterns of light absorption of an isolated pigment in solution at different wavelengths. These so-called absorption spectra (singular = spectrum) will allow you to identify which pigments you have isolated from the leaves.
Luteine

Zeaxantin
Violaxanthin

α-carotene

ß–carotene 
Antheraxanthin
Neoxanthin Figure 1. Molecular structures of carotenoids typically found in a leaf
Figure 1. Molecular structures of carotenoids typically found in a leaf
Images from:
http://commons.wikimedia.org/wiki/File:Luteine_-_Lutein.svg
http://commons.wikimedia.org/wiki/File:Zeaxantin.PNG
http://commons.wikimedia.org/wiki/File:Violaxanthin.svg
http://commons.wikimedia.org/wiki/File:Alpha-carotene.png
http://commons.wikimedia.org/wiki/File:Beta-carotene.png
http://commons.wikimedia.org/wiki/File:Antheraxanthin.svg
http://commons.wikimedia.org/wiki/File:Neoxanthin.svg
Chlorophyll

Figure 2. Molecular structures of chlorophylls a and b. Image from: http://commons.wikimedia.org/wiki/File:Chlorophyll_a_b_d.svg
In order to obtain an absorption spectrum one must first use a solvent to dissolve the chlorophylls and carotenoids from the thylakoids of the chloroplasts. In today’s lab, each pair of students will use 100% acetone to extract and identify the photosynthetic pigments from spinach leaves.
In addition to the chlorophylls and carotenoids you may also extract water-soluble anthocyanins, which are red or purple in color. These pigments are localized in the vacuole, and are not involved in light absorption for photosynthesis. It is not clear what role they play, but some evidence suggests that they protect leaves from harmful UV B radiation and deter herbivores due to their bitter taste.
The various pigments that you extract will be separated by making use of their differential polarity (which influences their solubility in various solvents) and by their differential rates of migration through a solid medium. In this case, migration will be up a sheet of filter paper. This method of separation is called paper chromatography.
The chromatography paper usually contains pure cellulose, which is a linear polymer of D-glucose with β-1,4 linkages and many polar side groups. The stationary phase refers to water that is tightly bound to the cellulose structure and fills the interspaces of the matrix (the paper fibers). The mobile phase refers to any solvent (known as the developing solvent) that is partially miscible (mixable) with water. Pigments that are highly water-soluble or that have the greatest hydrogen-bonding capacity will move more slowly along the paper, while less polar compounds travel faster with the developing solvent (petroleum ether, in this case). Paper chromatography is usually used for separating highly polar compounds such as sugars, amino acids, and some pigments.
The chromatography solution we shall use is a saturated solution of a polar solvent, n-propanol (1% vol/vol) in a relatively non-polar solvent, petroleum ether. As the solvent mixture ascends the 3MM filter paper (the matrix) by capillary action, the more polar component of the solvent adsorbs to the filter paper to become thousands of droplets of the stationary phase. These bound, stationary droplets are successively washed by the passing of the more non-polar, mobile phase. Those photosynthetic pigments having greater solubility in the more polar solvent will be retained or partitioned in the stationary phase, whereas other pigments more soluble in the non-polar, mobile phase will move up the chromatogram.
Once the pigments are separated on the chromatography paper according to their polarity, they can be extracted with a solvent. The identity and purity of the band can be tested by recording its absorption spectrum.
SPECTROPHOTOMETRY
Light is a form of electromagnetic radiation. It travels through space in rhythmic waves, and the distance between the crests of the waves is called the wavelength. Wavelengths of electromagnetic radiation range from less than a nanometer to greater than a kilometer, and this wavelength is inversely proportional to the amount of energy present. The entire range of electromagnetic radiation is called the electromagnetic spectrum, which is diagrammed in Figure 3.
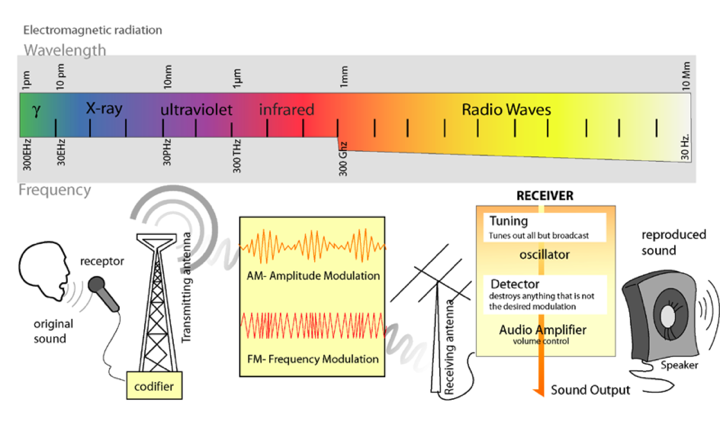
Figure 3. The Electromagnetic Spectrum. Image from: http://commons.wikimedia.org/wiki/File:Radio_transmition_diagram_en.png
The radiation in the visible portion of the spectrum is detected as colors by the human eye. Color is determined by the wavelengths of light that are reflected by molecules in the substance, while the other wavelengths are absorbed. Therefore, an object that appears red absorbs the blue-green wavelengths of light and reflects the red wavelengths.
Our perception of color is qualitative, but there are instruments that quantify the amount of light absorbed at a particular wavelength by a solution. These instruments are called spectrophotometers. As shown in Figure 4, a simple spectrophotometer contains a light source that is focused on a prism or diffraction grating, which splits this light into individual energy bands. These bands are further focused through a narrow slit, which can be moved across the spectrum to select a specific wavelength. This monochromatic (one wavelength) beam of light is passed through a cuvette containing the sample to be measured. As the light passes through the sample, some of it is absorbed by the molecules in the solution. The remainder of the light is transmitted through the sample, and impacts a photodetector that changes light energy into an electrical current. The magnitude of this current is proportional to the light intensity. The detector signal is amplified by a photomultiplier and is fed into the display meter as a number (digital) or a stylus deflection (analog), which indicates the amount of light absorbed by the molecules in the solution. Most spectrophotometers can also indicate the amount of light that is transmitted through the solution.

Figure 4. Diagram of the Light Paths in a Spectrophotometer. Image from:
http://commons.wikimedia.org/wiki/File:Spetrophotometer-en.svg
The Beer-Lambert Law
The amount of light that passes through a solution in a spectrophotometer is called transmittance (T), and can be defined mathematically as, T = I/Io, where I is the intensity of the light transmitted through the sample, and Io is the intensity of the incident light on the sample.
The absorbance (A) is the negative log of the transmittance, A = –log T. Absorbance is directly proportional to the concentration of the absorbing molecules in the solution and the distance the light travels through the solution. Therefore, absorbance can be used as a quantitative measure of the concentration of a solution. These concepts are expressed mathematically in the Beer-Lambert Law:

The molar extinction coefficient is a constant that is dependent upon the chemical nature of the absorbing material and the wavelength employed for the absorbance measurement. The path length is determined by the width or diameter of the cuvette used to contain the sample. Absorbance is unitless.
Quantitative analysis of many biological materials is based on: a) the material itself exhibiting light absorbance at a particular wavelength, or b) the biological material reacting with a chemical reagent to form a colored product in such a way that the absorbance of the product is a quantitative measure of the concentration of the biological material. Because these materials must be dissolved in a solvent before an absorbance reading can be obtained, absorption by the solvent is a potential source of error. To insure that the absorbance reading will reflect only the absorbance of the molecules to be quantified, spectrophotometers are initially set to zero absorbance with the solvent that was used to dissolve or dilute the solutions. The tube containing the solvent is called the blank.
PART I: Pigment Extraction and Separation
Half of the lab will begin with Part I and the other half of the students will begin with Part II.
Part I begins with extracting pigments from leaves and separation of the different pigments using paper chromatography.
All glassware used for pigment isolation and separation must be rinsed with 70% ethanol immediately after using it. CAUTION: Pour used solvents into the waste container in the HOOD. DO NOT POUR FLAMMABLE SOLVENTS INTO ANY SINK DRAIN. The solvents you will be using today: acetone, n-propanol and petroleum ether are all irritants to the eyes, skin and respiratory passages, so handle with care.
A. Extraction of Pigments (work in hood only)
- Extract photosynthetic pigments by grinding 2g of spinach leaves, torn into small pieces, in a mortar with a pinch of clean sand and a total of 10mL of 100% acetone. Initially, add only a small amount of acetone to begin the grinding process. It is much easier to grind the leaves if the extract is a pasty consistency. Add more solvent in small increments while continuing to grind the leaves. Pour the extract into a 15mL centrifuge tube and centrifuge in the benchtop centrifuge for 3min. Remove the extract to a 10mL beaker using a Pasteur pipette. Cover the beaker with Parafilm® to prevent evaporation. This extract will be used for chromatography.
- Clean-up: Wash mortar and pestle immediately with 70% ethanol. Discard ethanol in labeled waste bottle in hood. Discard centrifuge tube in hazard bag in hood.
B. Separation of Pigments by Paper Chromatography
- In the hood (wear gloves) Prepare 25mL of chromatography solvent by first adding 25mL of Petroleum ether into your chromatography jar followed by 250µL of n-propanol. Attach the lid tightly to allow saturation of gases in the jar. Label your jar with colored tape.
- Obtain a piece of 3MM filter paper, being very careful to hold it by the edges only. Place the paper on the covered table, and draw two dots with a pencil about 2cm from the bottom (narrow edge), and 1cm from the left and right edges. Do not use a pen, because pen marks will develop with the pigments.
- Using an eye-shadow applicator, apply the pigment extract between the dots in the manner demonstrated by your instructor. Do NOT extend the line to the side edges--leave 1cm space on each side. You should make ~3-5 applications of extract to obtain sufficient pigment in each band to result in good absorption spectra. Apply the extract with the edge of the applicator, not the wide face. This will give a thinner line. Wait ~ 30 seconds-1 min. between applications for the extract to dry, otherwise you will get a very thick line. The number of applications will depend on the type of plant used and the concentration of the extract. If the line is very faint, more applications are needed. Show your chromatogram to your instructor and let her/him judge if enough extract has been applied.
- Quickly, place the chromatogram in the chromatography jar in the hood, pigment line down and attach the lid tightly. Be sure the chromatography solvent (which contains petroleum ether with 1% n-propanol) in the jar will not stand higher than the line of applied pigments, that is, be sure the solution will migrate through the line and move the pigments up the paper rather than to diffuse them from the paper into the standing solvent.
- Observe as the solvent front moves up the paper. Stop the process when the solvent front is about 2 cm from the top. Note that the pigments move up the paper very quickly. How long does it take? Check after 5 minutes. At the end, there should be 3–5 bands of pigment: 2 green bands and 1–3 yellow bands. Generally, there is a yellow pigment band (migrating with the solvent front), followed by 2 green bands, and finally 0–2 yellow bands.
- Remove the chromatogram and allow it to dry for a minute in the hood. The pigments will evaporate so do not leave it in the hood too long.
C. Determination of Absorption Spectra
- With a pencil, number the separated pigments in your chromatogram from the top band (#1) to the application point (no movement). Use the lab's digital cameras to take a photo of your chromatogram. Download your photo onto the desktop of one of the lab computers ( Appendix J). Save it as a jpeg file. Save the file and email it to yourself and your partner in FirstClass. You can improve it later (crop, increase contrast, adjust image size etc) in Photoshop or iPhoto.
- Cut the chromatograms into horizontal strips as directed by your instructor. Take care to cut the strips so that they contain no more than one horizontal band of pigment. It is best to work with only one pigment at a time to avoid contamination of samples.
- Pipet 1mL of 100% acetone into three 13mm glass test tubes. Label the tubes by the fraction number from the chromatogram. Submerge each of the 3 types of pigments (yellow-green, blue-green and yellow-orange) separately into the solvent after cutting each strip into a couple of pieces that can be completely submerged in the solvent. Use your forcepts to remove and discard the paper after making sure all the pigment has been transfered to the solvent. Cover the opening of the tube with Parafilm® to prevent acetone evaporation. Pour acetone as a reagent blank into one of the glass cuvettes in your matched set until the cuvette is 2/3 to 3/4 full. Pour or use a Pasteur pipet to transfer the contents of one of the tubes of pigment to the other glass cuvette in your set. Bring your filled cuvettes to the Beckman spectrophotometer. Carry the cuvettes in a cuvette rack, as they are expensive. Do not dispose of their plastic tops! Refer to the directions for using the Beckman DU®530 spectrophotometer in Appendix C (Program #5, 400–700nm). If there is a line waiting for the spectrophotometers, cap your cuvettes or cover them with parafilm to prevent evaporation.
- Follow the directions in Appendix C. Run a baseline reading from 700–400nm, using the cuvette containing 100%. Then Run an absorption spectrum for each pigment, rinsing the sample cuvette with acetone (from a squirt bottle in the hood!) between readings. You may discard each pigment in the labeled container in the hood after running its absorbance spectrum. The peaks and valleys will be adjusted automatically by the spectrophotometer, by changing the range of % absorbance on the y-axis. You can use the cursor to obtain the wavelengths of the spectral peaks, or estimate them from the printed spectra. These peak wavelengths will be useful for determining the identities of the pigments associated with the spectra.
- When all the spectra have been recorded, print the chlorophylls together either on one graph or separately ( Appendix C). Print the carotenoids as a separate graph. Be sure to label which band from the chromatogram corresponds to each spectrum.
PART II: Spectrophotometery
Half of the class will begin with this portion of the lab, examining the absorption spectra of the separated pigments and determination of the concentration of chlorophyll a by generating a standard curve using a spectrophotometer.
Prepare A Serial Dilutions of Chlorophyll Derived Stock Solution Each pair will use a stock solution to prepare a series of 4 dilutions of a dietary supplement that contains chlorophyll among other compounds (stock chlorophyll derived compounds at 0.05mg/ml). Each dilution in the series will differ from the previous dilution by 1/2, so the dilution ratio for each tube is 1/2, 1/4, and 1/8. Calculate the concentrations of the chlorophyll derivative that the tubes (in micrograms/ml) will contain after the dilutions have been prepared, and record them in your lab notebook.
- Label four 13mm test tubes with the dilution ratios: 1/2, 1/4, 1/8, 1/16.
- With a 5.0mL serological pipette add 4.0mL distilled water to each tube.
- Using a clean pipette, add 4.0mL of stock solution to the tube labeled 1/2 and mix by inversion (not vortexing). Use square of Parafilm® to cover test tube opening. Keeping thumb on Parafilm®, invert test tube twice.
- Use a clean pipette to add 4.0mL of 1/2 dilution to the tube labeled 1/4; mix.
- Use a clean pipette to add 4.0mL of 1/4 dilution to the tube labeled 1/8; mix.
- Use a clean pipette to add 4.0mL of 1/8 dilution to the tube labeled 1/16; mix.
B. Determination of Absorbance versus Concentration
You will now use all four dilutions (stock, 1/2, 1/4, 1/8, 1/16) to demonstrate the relationship between absorbance and concentration. Do not read the stock solution, as this will give you an absorbance value that is too high. Set the Spectronic20+® spectrophotometer to the wavelength that gave maximal absorption for chlorophyll a. (You can determine the best wavelength to use in your measurements from the reference spectra your instructor has available for you to consult OR from the peak absorbance of chlorophll a that you determined from your wavelength scan of your blue-green pigment fraction.) Zero the instrument using water as the blank( Appendix B). Read the absorbance of the four concentrations and record them in your lab notebook next to the appropriate concentrations. Using the Excel directions in Appendix D plot these data to generate a simple scatter plot. Be sure to label the axes and give units, if appropriate, (mg/ml or ug/ml on the x and A no units on the Y). Is there any apparent relationship between the absorbance values and the concentrations of the four solutions? Does this relationship follow the Beer-Lambert Law?
C. Data Analysis and Determination of an Unknown Concentration of Chlorophyll Derived Solution
This section of the lab will address some of the ways that analytical data are presented and evaluated. For this, you will be using the absorbance and concentration values obtained for your chlorophyll derived solutions in Part C.
Linear Regression Analysis
Linear Regression is a method of data evaluation that enables us to create a linear plot of absorbance data versus concentration for a series of solutions. This plot can be used as a measure of compliance with the Beer-Lambert Law and to predict the concentrations of unknown samples. The plot generated by the linear regression method is called a standard curve, and is described by the equation y = mx + b, where m is the slope of the line, b is the y intercept, and x, y are the data points. Because all data may not be perfectly linear, a measure of how well the regression line fits the actual data points, called the correlation coefficient (R2), is also determined. The calculations for linear regression are quite tedious, so computer programs are often used to assist with the calculations. We will be using Microsoft Excel to calculate and plot linear regression data today. The data must be entered such that the independent variable (concentration, in this case) is plotted on the x-axis. The dependent variable (absorbance, in this case) will be displayed on the y-axis. A sample standard curve can be found here: Dunne RP (1999) Spectrophotometric measurement of chlorophyll pigments: a comparison of conventional monochromators and a reverse optic diode array design. Mar Chem 66:245-251.
The linear regression equation and the R2 value are printed on the graph. A R2 value equal to 1 is indicative of perfect correlation among all the data points, and R2 values of 0.99-0.97 indicate good linearity. If acceptable linearity is demonstrated, the regression equation can be used to calculate the concentrations of unknown solutions by substituting the absorbance of the solution into the equation as the y value and solving for x.
To verify the linearity of your dilution series data, use Excel ( Appendix E) for regression plots) to plot a standard curve using the concentrations and corresponding absorbance values for these solutions determined in Part B. Is your standard curve linear? If so, what does this tell you about the relationship between concentration and absorbance? If your standard curve is not linear, consider the types of errors that may have contributed to this lack of linearity.
Determination of Unknown Concentration
There is an animation showing how to determine the concentration of an unknown. Your lab instructor will give you a test tube with an unknown concentration of chlorophyll derived solution. At the appropriate wave length, measure the absorbance and determine the concentration of this solution. Before you leave the lab, please hand in your standard curve along with the measured chlorophyll derived solution's concentration.
Laboratory Cleanup
- Wash glassware with 70% ethanol as soon as possible.
- Discard all ethanol in labeled bottle in hood.
- Place glass pipettes tips down in pipette canister.
- Discard Pasteur pipettes in red sharps container.
- Rinse cuvettes with 95% ethanol and leave upright to dry.
- Discard eye-shadow applicator.
Assignment
Turn in at the beginning of Lab 9
1. Write a results section (data analysis) for your Part I work today, answering the question, Which specific photosynthetic pigments in spinach leaves are identified by partition chromatography? This analysis should include a composite figure made from either an overlay graph of your absorbance spectra OR from making a composite figure showing EACH of your separate spectra as parts of a single figure (with an identification system for the parts that makes it clear which spectrum comes from which numbered fraction on your chromatogram). You will need to scan the spectra to get digital versions for this assignment. There are scanners in the Science Library and in the Knapp Center. Present your evidence in figures in a logical sequence. DO NOT include the standard curve since this results section omits the part of the lab that involved creating the standard curve and using it to calculate the concentration of an unknown. All figures should have appropriate figure legends.
The narrative portion of this results analysis should start with the experimental goals (In order to discover....) and then briefly give a general description of the experimental methods employed to answer the question addressed. This is results and not materials and methods, so do NOT include a step by step description of your procedure with details like volumes of reagents, etc. Figures should be inserted at the end of the paragraph that introduces the figure directly by figure number. Since you have labeled the pigments on the chromatograph as 1, 2, etc., the figure legend should include a key of answers to the identity of the numbered separated pigments and reference the spectra figure to explain how the identities were determined. The analysis narrative will, necessarily, include references to outside sources of information for reference peak absorbance spectra of pigments and for the chemical structure of the pigments that accounts for their differences in polarity and migration in your chromatography solvents. Your evidence for your conclusions about the ID the photosynthetic pigments is made from a combination of comparisons of your measured peak absorbances to known spectra, relative mobility due to chemical structural differences that cause differences in polartiy, and from transmitted color. Be sure to include ALL of those parameters (chemical structure and polarity related to migration, transmitted color, and peak absorbance) in your identification analysis.
2. In your lab notebook, prepare a flow chart for the Hill Reaction (Lab 9).
3. Review the mechanisms of electron transport and proton flow in photosynthesis in your textbook.
Link to Labs in this Series and the Last Series
Lab 8: Taster Data Analysis & Science Writing Workshop; Spectrophotometry and Photosynthesis 1
Lab 9: The Hill Reaction
Lab 10: Variables Affecting the Hill Reaction
Lab 11 Gene Regulation/Enzymology

