BISC110/S12: Lab 8: Difference between revisions
| Line 5: | Line 5: | ||
=='''Introduction to Series 3--Spectrophotometry'''== | =='''Introduction to Series 3--Spectrophotometry'''== | ||
'''SPECTROPHOTOMETRY''' | '''SPECTROPHOTOMETRY''' | ||
Light is a form of electromagnetic radiation. It travels through space in rhythmic waves, and the distance between the crests of the waves is called the wavelength. Wavelengths of electromagnetic radiation range from less than a nanometer to greater than a kilometer, and this wavelength is inversely proportional to the amount of energy present. The entire range of electromagnetic radiation is called the electromagnetic spectrum, which is diagrammed in Figure 3.<br><br> | Light is a form of electromagnetic radiation. It travels through space in rhythmic waves, and the distance between the crests of the waves is called the wavelength. Wavelengths of electromagnetic radiation range from less than a nanometer to greater than a kilometer, and this wavelength is inversely proportional to the amount of energy present. The entire range of electromagnetic radiation is called the electromagnetic spectrum, which is diagrammed in Figure 3.<br><br> | ||
Revision as of 09:32, 22 March 2012
Part 1: Spectrophotometry and Creating a Standard Curve
Your lab today is divided into two distinct parts. In the later portion of the lab, you will participate in a writing workshop for the data collected in labs 6 and 7. In the first portion of the lab, there will be a wet lab component where you will perform a wavelength spectrum of a sample to determine the wavelength at which the sample has its maximum absorbance. You will use several dilutions of the sample solution to measure the absorbance of the diluted samples at the wavelength obtained from the spectrum and you will use this data to generate a standard curve. This will help you to calculate the concentration of an unknown sample.
Introduction to Series 3--Spectrophotometry
SPECTROPHOTOMETRY
Light is a form of electromagnetic radiation. It travels through space in rhythmic waves, and the distance between the crests of the waves is called the wavelength. Wavelengths of electromagnetic radiation range from less than a nanometer to greater than a kilometer, and this wavelength is inversely proportional to the amount of energy present. The entire range of electromagnetic radiation is called the electromagnetic spectrum, which is diagrammed in Figure 3.
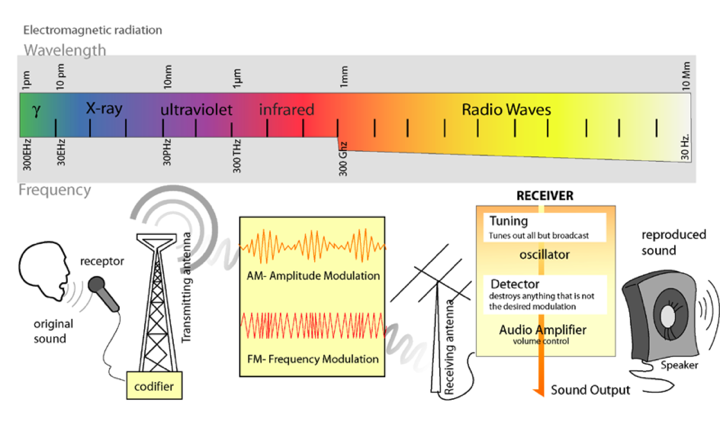
Figure 3. The Electromagnetic Spectrum. Image from: http://commons.wikimedia.org/wiki/File:Radio_transmition_diagram_en.png
The radiation in the visible portion of the spectrum is detected as colors by the human eye. Color is determined by the wavelengths of light that are reflected by molecules in the substance, while the other wavelengths are absorbed. Therefore, an object that appears red absorbs the blue-green wavelengths of light and reflects the red wavelengths.
Our perception of color is qualitative, but there are instruments that quantify the amount of light absorbed at a particular wavelength by a solution. These instruments are called spectrophotometers. As shown in Figure 4, a simple spectrophotometer contains a light source that is focused on a prism or diffraction grating, which splits this light into individual energy bands. These bands are further focused through a narrow slit, which can be moved across the spectrum to select a specific wavelength. This monochromatic (one wavelength) beam of light is passed through a cuvette containing the sample to be measured. As the light passes through the sample, some of it is absorbed by the molecules in the solution. The remainder of the light is transmitted through the sample, and impacts a photodetector that changes light energy into an electrical current. The magnitude of this current is proportional to the light intensity. The detector signal is amplified by a photomultiplier and is fed into the display meter as a number (digital) or a stylus deflection (analog), which indicates the amount of light absorbed by the molecules in the solution. Most spectrophotometers can also indicate the amount of light that is transmitted through the solution.

Figure 4. Diagram of the Light Paths in a Spectrophotometer. Image from:
http://commons.wikimedia.org/wiki/File:Spetrophotometer-en.svg
The Beer-Lambert Law
The amount of light that passes through a solution in a spectrophotometer is called transmittance (T), and can be defined mathematically as, T = I/Io, where I is the intensity of the light transmitted through the sample, and Io is the intensity of the incident light on the sample.
The absorbance (A) is the negative log of the transmittance, A = –log T. Absorbance is directly proportional to the concentration of the absorbing molecules in the solution and the distance the light travels through the solution. Therefore, absorbance can be used as a quantitative measure of the concentration of a solution. These concepts are expressed mathematically in the Beer-Lambert Law:

The molar extinction coefficient is a constant that is dependent upon the chemical nature of the absorbing material and the wavelength employed for the absorbance measurement. The path length is determined by the width or diameter of the cuvette used to contain the sample. Absorbance is unitless.
Quantitative analysis of many biological materials is based on: a) the material itself exhibiting light absorbance at a particular wavelength, or b) the biological material reacting with a chemical reagent to form a colored product in such a way that the absorbance of the product is a quantitative measure of the concentration of the biological material. Because these materials must be dissolved in a solvent before an absorbance reading can be obtained, absorption by the solvent is a potential source of error. To insure that the absorbance reading will reflect only the absorbance of the molecules to be quantified, spectrophotometers are initially set to zero absorbance with the solvent that was used to dissolve or dilute the solutions. The tube containing the solvent is called the blank.
Determination of an Absorption Spectrum
- In pairs, obtain two 13mm glass test tubes from the stock area in the front of the lab.
- Label one test tube "Sample" and the other "BLANK".
- With a 10.0 mL serological pipette add 6.0 mL distilled water to the "BLANK" tube and add 8.0 ml of the sample solution to the tube marked "Sample".
- Obtain two disposable plastic cuvettes and fill one with water and the other with the sample so that each of them are about 3/4 full. The exact amount is not important. It is not necessary to label these cuvettes; you will be able to tell the difference by color of the contents.
- Follow the directions in Appendix C for using a BeckmanDU530 or 720 spectrophotometer to run an absorbance spectrum. First run a baseline reading from 400-700nm, using a cuvette filled with water. Run an absorption spectrum for the sample. After the spectrum is complete the peaks and valleys will be rescaled automatically as the instrument adjusts the range of % absorbance on the y-axis. When it stops reading and adjusting, you can use the cursor to obtain the wavelengths of the spectral peaks.
Determining Concentration from Absorbance
A. Prepare Serial Dilutions of Sample
Each pair will use a stock solution to prepare a series of 4 dilutions. The substance that shows a maximum absorbance at the wavelength you determined is present at a concentration of 0.05 mg/ml.
- Label four 13mm test tubes with the dilution ratios: 1/2, 1/4, 1/8, 1/16.
- With a 5.0mL serological pipette add 4.0mL distilled water to each tube.
- Using a clean pipette, add 4.0mL of stock solution to the tube labeled 1/2 and mix by inversion (not vortexing). Use square of Parafilm® to cover test tube opening. Keeping thumb on Parafilm®, invert test tube twice.
- Use a clean pipette to add 4.0mL of 1/2 dilution to the tube labeled 1/4; mix.
- Use a clean pipette to add 4.0mL of 1/4 dilution to the tube labeled 1/8; mix.
- Use a clean pipette to add 4.0mL of 1/8 dilution to the tube labeled 1/16; mix.
- Calculate the final concentrations of each of the tubes using the stock concentration of 0.05 mg/ml and record them in your lab notebook.
B. Determination of Absorbance
Set the Spectronic20+® spectrophotometer to the wavelength at which the sample show the maximum absorbance from the wavelength spectrum scan. Zero the instrument using water as the blank( Appendix B). Read the absorbance of the four concentrations and record them.
C. Generation of a Standard Curve and Determination of the Concentration of an Unknown Solution
Using the Excel directions in Appendix D plot these data to generate a simple scatter plot. Be sure to label the axes and give units, if appropriate, (mg/ml or ug/ml on the x and A no units on the Y). Is there any apparent relationship between the absorbance values and the concentrations of the four solutions? Does this relationship follow the Beer-Lambert Law?
Linear Regression Analysis
If the data show an apparent proportional relationship, one would fit the data with a linear regression. Excel will provide an equation for the model. Linear Regression is a method of data evaluation that enables us to create a plot of absorbance data versus concentration for a series of solutions. The plot generated by the linear regression method is called a standard curve, and is described by the equation y = mx + b, where m is the slope of the line, b is the y intercept, and x, y are the data points. A measure of how well the regression line fits the actual data points, called the correlation coefficient (R2), is also determined. A R2 value equal to 1 is indicative of perfect correlation among all the data points, and R2 values of 0.99-0.97 indicate good linearity. If acceptable linearity is demonstrated, the regression equation can be used to calculate the concentrations of unknown solutions by substituting the absorbance of the solution into the equation as the y value and solving for x.
If your standard curve is not linear, consider the types of errors that may have contributed to this lack of linearity.
Determination of Unknown Concentration
There is an animation showing how to determine the concentration of an unknown. Your lab instructor will give you a test tube with an unknown concentration of sample solution. At the appropriate wave length, measure the absorbance and determine the concentration of this solution. Before you leave the lab, please hand in your standard curve along with the calculated concentration of the unknown sample.
Laboratory Cleanup
- Place glass pipettes tips down in pipette canister.
- Discard Pasteur pipettes in red sharps container.
- Discard glass test tubes (disposable) in glass discard box in front of lab.
Part 2: Taster Data Analysis & Science Writing Workshop
In the second part of lab, you will participate in a peer review of the drafts of the figures and a results and discussion section that you prepared for homework. Your instructor will conduct a writing workshop and discuss expectations for the paper due in lab 9.
Assignment
Turn in at the beginning of Lab 9
1. Your completed TASTER paper is due at the beginning of Lab 9. Please write a partial scientific paper (Title, Abstract, Results, Discussion, and Literature Cited). Your instructor will provide more detailed instructions during your writing workshop.
2. In your lab notebook, prepare a flow chart for the Hill Reaction (Lab 9).
3. Review the mechanisms of electron transport and proton flow in photosynthesis in your textbook.
