BISC209/F13: Lab5
LAB 5: Culture Independent Survey of Bacteria in a Soil Community by Illumina™ 16S rRNA gene sequencing
Background
In the 1980's scientists discovered that, despite the unicellular character of microorganisms and their invisibility to us, the microbial world is much more diverse and numerous than the macroscopic world of plants and animals. We now know that microbial communities are as complex and interdependent as those of visible forms of life. Traditional measures of diversity relied on physical traits and assessment of microbial metabolism was used for microbial identification rather than for understanding community structure and organization. The use of physical traits in determining relationships between organisms has several major problems when it comes to microbes: 1) microbes are not as morphologically diverse as metazoans; 2) their morphological traits are not indicative of evolutionary relationships; 3) there are no morphological traits common to both macroorganisms and microbes. In the 1980's Carl Woese suggested that the deoxyribonucleic acid (DNA) sequences of certain common genes could be used to measure relatedness among radically different organisms. He picked the genes that encode ribosomal RNA (rRNA). Ribosomes, the protein-RNA complexes that are the scaffold on which proteins are synthesized, are common to all cells, both prokaryotic and eukaryotic. Despite differences in size, the sequences of rRNA molecules contain regions that are highly conserved, thus highly similar. Woese chose the gene for the intermediate sized rRNA molecule, 16S rRNA in prokaryotes and 18S rRNA in eukaryotes, because this gene was large enough to contain enough information for genetic comparisons but small enough for the gene to be sequenced easily.
Comparing sequences of the gene (16S DNA) that encodes 16S rRNA in different bacteria can be used to identify them with much greater accuracy than traditional identification tools that used a battery of metabolic and physical tests on cultured community members. It is also common now to use rDNA sequencing to deduce phylogenetic relationships between different bacteria and among organisms as diverse as bacteria and humans. Woese's ground-breaking work altered the tree of life and showed that the prokaryotic world was evolutionarily much older than expected and much more important.
Today you will begin a culture independent analysis of the bacteria in your soil community by next-generation, high-throughput 16S ribosomal RNA gene sequencing. This gene encodes ribosomal RNA that is specific to all prokaryotes (bacteria) and is an excellent phylogenetic marker for bacteria. It has been widely used in surveys of microbial community composition and in bacterial identification.
We will use the bacterial primers to amplify the conserved part of the 16S rRNA gene, called 27 Forward (27F) and 1492 Reverse (1492R). The numbers refer to positions on the Escherichia coli 16S rRNA gene. The direction of elongation of new DNA can only proceed in the 5' to 3' direction; therefore, the primer that anneals to base 27 on the 16S rRNA gene template is called the forward primer because DNA synthesis moves downstream on the template. The 1492R primer anneals to base 1492 on the opposite template strand and DNA synthesis moves upstream from the template. Our primers are called "universal" bacterial primers because they anneal to a 16S rRNA region that is highly similar (conserved) to most Bacteria. If we were interested in identifying eukaryotic microbes, such as fungi or protozoa, or if we wanted to identify the Archaea (which are neither Eukarya or Bacteria), we would have to use different primers.
Although our primers have sequences of single stranded DNA that are common to the 16S rRNA gene of most bacteria, the section of the 16S rRNA gene that will be amplified by the DNA polymerase contains highly variable regions of that gene. So variable, in fact, that we should be able to use the DNA sequence of those ~1,500 bases to identify our soil bacteria by comparing the sequences we amplify to the same region of the gene in other bacteria. 16S rRNA gene sequences are archived in an extensive, public database called the Ribosomal DNA Project (RDP) found at | http://rdp.cme.msu.edu/index.jsp;jsessionid=04A058D492CD00120AC01A70AAD7624A . There are so many 16S rRNA gene sequences archived at this site that we should be able to use their powerful, public search engine to identify our soil bacteria down to the genus or species level, if our pcr and the sequencing of the gene from the clones all works well.
Part A: Isolation of Genomic DNA From a Soil Sample
To start the culture independent bacterial identification (sequencing 16S rRNA genes), each student will need a 0.25 gram sample of sieved soil. Weigh out your aliquots from the freshly collected soil that one of your partners collected before lab. Use the top loading balance and wieghing paper that you are careful not to contaminate. Fold up the edges of the weighing paper to use as a crude funnel and pour the 0.25g of soil into a special PowerBead tube from the MoBIO PowerSoil DNA isolation kit (these tube will be at the instructor's bench). Make sure you use the right tube and that your soil isn't too wet to start the isolation. (See the Wet Soil option of the Soil DNA isolation protocol below.) A genomic DNA soil isolation will be performed in one Powerbead tube, using soil from the freshly collected sample.
Protocol for Using the MO BIO laboratories, Inc. Power® Soil DNA Isolation Kit (cat. # 12888-50).'
Manufacturer Information at | http://www.mobio.com
visual protocol at [1]

Please wear gloves during this protocol
Wet Soil Sample Option
If soil sample is dry or slightly damp you may skip this '"Wet Sample Option'" and start directly with step 1 below. If soil sample is muddy or really wet (this is unlikely), consult with your instructor. You may need to remove the contents from the from PowerBead Tube (beads and solution) and transfer them into another sterile microcentrifuge tube. Add your soil sample to PowerBead Tube and centrifuge at room temperature for 30 seconds at 10,000 x g. Remove as much liquid as possible with a pipet tip. Add beads and bead solution back to PowerBead Tube and follow protocol starting at step 2.
Dry Soil Sample
1. Each student will do the DNA isolation. You will use one 0.25 g soil sample. Add the soil to a PowerBead Tube and label your tube on the top only with your initials and a soil sample identifier (your site code and initial(s) on a piece of your team color tape.
What’s happening: After your sample has been loaded into the PowerBead Tube, the next step is a homogenization and lysis procedure. The PowerBead Tube contains a buffer that will (a) help disperse the soil particles, (b) begin to dissolve humic acids and (c) protect nucleic acids from degradation.
2. Gently vortex to mix.
What’s happening: Gentle vortexing mixes the components in the PowerBead Tube and begins to disperse the sample in the PowerBead Solution.
3. Check Solution C1 to see that it's not precipitated. If Solution C1 is precipitated, heat solution to 60°C until the precipitate has dissolved before use.
What’s happening: Solution C1 contains SDS and other disruption agents required for complete cell lysis. In addition to aiding in cell lysis, SDS is an anionic detergent that breaks down fatty acids and lipids associated with the cell membrane of several organisms. If it gets cold, it will form a white precipitate in the bottle. Heating to 60C will dissolve the SDS and will not harm the SDS or the other disruption agents. Solution C1 can be used while it is still warm.
4. Add 60 μL of Solution C1 to your PowerBead tube and invert several times or vortex briefly.
5. Tape your tubes to the Fisher vortex Genie2 so that the tubes lie horizontally  . The tubes need to be securely held by the tape and the tape needs to be attached to the vortex, allow the tape to be loose enough so that the gasket will vibrate easily
. The tubes need to be securely held by the tape and the tape needs to be attached to the vortex, allow the tape to be loose enough so that the gasket will vibrate easily ![]() . Keep a watch to make sure the tubes will not fly off while being vortexed. Please wait until at least 2 samples are ready to votex and then turn it on to full power. One vortex can hold up to 4 tubes (two tubes on top and two on the bottom). Your samples will "turbovortex" for 10 minutes.
. Keep a watch to make sure the tubes will not fly off while being vortexed. Please wait until at least 2 samples are ready to votex and then turn it on to full power. One vortex can hold up to 4 tubes (two tubes on top and two on the bottom). Your samples will "turbovortex" for 10 minutes.
What’s happening: This step is critical for complete homogenization and cell lysis. Cells are lysed by a combination of chemical agents from steps 1-4 and mechanical shaking introduced at this step. By randomly shaking the beads in the presence of disruption agents, collision of the beads with microbial cells will cause the cells to break open.
6. Microcentrifuge your tubes at 10,000 rcf for 1 minute at room temperature. Caution: Be sure not to exceed 10,000 rcf or the tubes may break. Make sure the PowerBead tubes rotate freely in your centrifuge without rubbing!
7. Transfer the supernatant (don't transfer the beads!) to a clean 2 ml Collection Tube at the instructor's desk. If you don't know what a collection tube is, ask your instructor. Don't use a regular microfuge tube.
Note: Expect between 400 to 500 microliters of supernatant at this step. The exact recovered volume depends on the absorbancy of your starting material and is not critical for the procedure to be effective. The supernatant may be dark in appearance and still contain some soil particles. The presence of carry over soil or a dark color in the mixture is expected in many soil types at this step.
Subsequent steps in the protocol will remove both carry over soil and coloration of the mixture.
8. Add 250 μL of Solution C2 to the collection tube and vortex for 5 seconds. Incubate at 4°C for 5 minutes.
What’s happening: Solution C2 contains a patented reagent to precipitate non-DNA organic and inorganic material including humic substances, cell debris, and proteins. It is important to remove contaminating organic and inorganic matter that may reduce DNA purity and inhibit downstream DNA applications.
9. Centrifuge the Collection Tube at room temperature for 1 minute at 10,000 rcf.
10. Avoiding the pellet, transfer up to, but no more, than 600 microliters of supernatant to a clean 2 ml Collection Tube (provided).
What’s happening: The pellet at this point contains non-DNA organic and inorganic material including humic acid, cell debris, and proteins. For the best DNA yields, and quality, avoid transferring any of the pellet.
11. Add 200 microliters of Solution C3 and vortex briefly. Incubate on ice for 5 minutes.
What’s happening: Solution C3 is a second reagent (patented) to precipitate additional non-DNA organic and inorganic material including humic acid, cell debris, and proteins. It is important to remove contaminating organic and inorganic matter that may reduce DNA purity and inhibit downstream DNA applications.
12. Centrifuge the tube at room temperature for 1 minute at 10,000 rcf.
13. Avoiding the pellet, transfer up to, but no more, than 750 µL of supernatant to a clean 2 mL Collection Tube (provided).
What’s happening: The pellet at this point contains additional non-DNA organic and inorganic material including humic acid, cell debris, and proteins. For the best DNA yields, and quality, avoid transferring any of the pellet.
14. Shake to mix Solution C4 before use. Add 1.2 mL (do this by adding 600 µL twice) of Solution C4 to the supernatant (be careful solution doesn’t exceed rim of tube) and vortex for 5 seconds.
What’s happening: Solution C4 has a high concentration of salts. Since DNA binds tightly to silica at high salt concentrations, this will adjust the DNA solution salt concentrations to allow binding of DNA, but not non-DNA organic and inorganic material that may still be present at low levels, to the Spin Filters.
15. Load approximately 675µL of the C4 + supernatant mixture from the previous step onto a Spin Filter sitting in a Collection Tube (save the remainder of the supernatant!!) and centrifuge the spin filter at 10,000 rcf for 1 minute at room temperature. Discard the flow through (NOT the spin filter!!!) and put the spin filter back in the Collection Tube. Add an additional 675 µL of the Step 14 mixture to the same Spin Filter and centrifuge at 10,000 rcf for 1 minute at room temperature. Discard the flow through and load the remainder of the Step 14 mixture onto the Spin Filter in the Collection Tube and centrifuge at 10,000 rcf for 1 minute at room temperature.
Note: A total of three loads for each sample processed are required. You will using the same Spin Filter and Collection Tube for all 3 spins.
What’s happening: DNA is selectively bound to the silica membrane in the Spin Filter device in the high salt solution. Contaminants pass through the filter membrane, leaving only DNA bound to the membrane.
16. Add 500 µL of Solution C5 to the Spin Filter in the Collection Tube and centrifuge at room temperature for 30 seconds at 10,000 rcf.
What’s happening: Solution C5 is an ethanol based wash solution used to further clean the DNA that is bound to the silica filter membrane in the Spin Filter. This wash solution removes residual salt, humic acid, and other contaminants while allowing the DNA to stay bound to the silica membrane.
17. Discard the flow through (not the Spin Filter) from the 2 mL Collection Tube.
What’s happening: This flow through fraction is just non-DNA organic and inorganic waste removed from the silica Spin Filter membrane by the ethanol wash solution.
18. Centrifuge the Spin Filter in the Collection Tube again at room temperature for 1 minute at 10,000 rcf.
What’s happening: This second spin removes residual Solution C5 (ethanol wash solution). It is critical to remove all traces of wash solution because the ethanol in Solution C5 can interfere with many downstream DNA applications such as PCR, restriction digests, and gel electrophoresis.
19. Carefully place Spin Filter in a clean 2 mL Collection Tube (provided). DO NOT transfer any liquid that may be on the bottom of the spin filter basket and avoid splashing any Solution C5 onto the Spin Filter.
Note: It is important to avoid any traces of the ethanol based wash solution in the elution that will be created in the next step.
20. Add 35 μL of Solution C6 to the center of the white Spin Filter membrane.
Note: Placing the Solution C6 (sterile elution buffer) in the center of the small white membrane will make sure the entire membrane is wetted. This will result in a more efficient and complete release of the DNA from the silica Spin Filter membrane. As Solution C6 (elution buffer) passes through the silica membrane, DNA that was bound in the presence of high salt is selectively released by Solution C6 (10 mM Tris) which lacks salt.
Alternatively, sterile DNA-Free PCR Grade Water may be used for DNA elution from the silica Spin Filter membrane at this step (MO BIO Catalog# 17000-10). Solution C6 contains no EDTA. If DNA degradation is a concern, Sterile TE may also be used instead of Solution C6 for elution of DNA from the Spin Filter.
21. Centrifuge the Spin Filter in its Collection Tube at room temperature for 30 seconds at 10,000rcf.
22. Discard the Spin Filter. Save the eluent with the DNA.
23. Storing DNA: The DNA in the collection tube is now eluted and ready for use as a PCR template after we assess its concentration. The DNA eluted in Solution C6 (10 mM Tris) must be used immediately or stored at -20 °C to -80 °C to prevent degradation.
24. Transfer 10 µL of this Genomic DNA to a 200 µL microfuge tube. Label both the collection tube of eluted DNA and the microfuge tube containing 10 µL of the Genomic DNA with your initials, site letter, and the words GENOMIC, leave room to add the concentration you will get after using the nanodroppe.
25. Make sure your 2 tubes of DNA are labeled and on ice before you go with your instructor to the nanodropper to find out the concentration of the genomic DNA. We expect concentrations of DNA to be about 10-30 ng/µL. Add the concentration (ng/µL) to the tubes you labeled in step 24.
Part B: Measuring the Concentration of DNA
The Nanodropper is located in the BISC Equipment room, L308. Both the ThermoScientific NanoDrop 2000 and The NanoDrop ND-1000 Spectrophotometers measure DNA by taking Absorbance at A260nm. These spectrophotometers use only 1 microliter of sample and do not require cuvettes. The sample is held in place by fiber optic technology and surface tension that holds the sample in place between two optical surfaces that define the pathlength vertically and dynamically. Measurement can be assessed in a range of 2 to 3700 nm/microliter dsDNA. These are expensive machines so make sure you follow the directions carefully and ask your instructor for guidance as needed.
More information is available from the manufacturer's website at: | http://www.nanodrop.com/HowItWorks.aspx

Using the Nanodroppers
1. Clean the upper and lower optical surfaces of the sample retension device by pipetting 2 microliters of clean deionized water onto the lower optical surface. Close the lever arm and tap it a few times to bathe the upper optical surface. Lift the lever arm and wipe off both optical surfaces with a Kimwipe.

2. Open the NanoDrop software from the Desktop of the computer and select the nucleic acids module.
3. Initialize the machine by placing 1 microliter of clean deionized water onto the lower optic surface, lower the lever arm, and select initialize from the NanoDrop software. Once initialization is complete (~10sec.), clean both optical surfaces with a Kimwipe.

4. Perform a blank measurement by loading 1 microliter of Solution 6 (10mM Tris) and select Blank. Note that this blanking step may use something other than Tris depending on what your sample is dissolve in. Often the blank will be deionized water if you have concentrated your DNA sample already with the ethanol precipation and resolubilized it in water.
Note that as in traditional spectroscopy, the blank will be subtracted from subsequent measurements. If you want to determine the contribution of a specific buffer or diluent, measure the buffer first using distilled water as a blank. If the buffer does not contribute to the A 260nm reading, then deionized water will be fine to use as the blank. The water or buffer should always be measured to be sure that the instrument has been zeroed properly. The measurement of water or buffer should be zero or very close. All measurements are automatically normalized to 340nm.
5. Measure the nucleic acid sample by loading 1microliter of sample and selecting "measure". Record your DNA concentration. Once the measurement is complete. Clean both optical surfaces with a Kimwipe and the machine is ready for the next sample.
You should ensure that the appropriate constant (50 for dsDNA or 40 for RNA) has been chosen. The software automatically calculates the nucleic acid concentration. If the calculation is done by hand, the A260nm is represented as a 1cm path for convenience, even though 1 nm and 0.2nm paths are actually used during the measurement cycle.
Clean Up
When the last sample was been measured, clean the sampling device by repeating step 1.
PART C: PCR AMPLIFICATION of 16S rRNA gene
If you would like to review the process of DNA amplification by polymerase chain reaction, the link below will take you to the Dolan DNA center's video on the process, | http://www.dnalc.org/resources/animations/pcr.html
Polymerase chain reaction amplification of DNA requires a heat stable DNA polymerase. There are many choices. We will use a proof-reading polymerase called TaKaRa Ex Taq™ from a manufacturer called TaKaRa. Specificity primers, short sequences of DNA that are complementary to a small section of the DNA coding for 16S rDNA, will bind to the template strands extracted from the soil. The primers that we will use to amplify the 16S rRNA gene are called "Universal" Bacterial Primer sequences. They are "universal" because all eubacteria (prokaryotes) have some form of this gene that is recognized by these two primers. They are named for the base on this gene to which the primer anneals. Our universal primers are 1492R reverse primer TACGG(C/T)TACCTTGTTACGACTT and forward primer 27F AGAGTTTGATC(C/A)TGGCTCAG. Note that the () indicates degeneracy, where either of two bases can be used. Our primers are purchased from Integrated DNA Technologies (IDT).
Setting up the PCR Mix
WEAR GLOVES AT ALL TIMES AND DON'T TOUCH THE INSIDE OF THE TUBE CAPS OR YOUR PIPET TIPS--Always use a new tip when going into anything in a PCR reaction. (Contamination is a significant problem in PCR)
All reagents for the PCR should be kept on ice and the master mix should be thawed on ice. Since DNA polymerase can function at room temp, we don't want the reaction to start until all the tubes are in the thermal cycler.
The components below have been aliquoted and prepared for you and are in PCR color coded PCR tubes. Label a PCR tube with a fine point Sharpie on the top and side of the tube with a unique identifier. We will set up one tube per lab as a neg. control and one tube per lab as a pos. control.
Using a P2 or P10 and filter tips (remember that the P2 has two red decimal place volume indicators while the P10 only has 1 red decimal place indicator. MAKE SURE YOU HAVE DIALED IN THE CORRECT VOLUME!), add 2 µL of the correct concentration of your environmental soil sample DNA to the pre-aliquoted 48 µL of Master Mix (contains DNA polymerase, dNPTs, MgCl2, and buffers), primers and nuclease free water mixture described above (for a total volume of 50 μL) in clearly labeled PCR tubes of your team color. Make sure you label on both the top and sides of the tube. The tubes are tiny (holds only 0.2 ml or 200 µL vol) and it is hard to write on them legibly but doing so is very important. You will have to use a unique identification code and keep the key to the code in your lab notebook (also give a copy to your instructor).
For the negative control, one person in the lab will add 2µL water in place of the template DNA.
For the positive control, one person in the lab will add 1 µL of water and 1 µL of E. coli DNA extract. When you have mixed your DNA or water into the PCR mix by tapping VERY LIGHTLY or flicking to be sure that all reagents are mixed and not adhering to the tube wall, spin briefly, and take your tubes to the thermal cycler when your instructor says it's ready. Keep them on ice until then, but wipe off the bottom of the tubes before putting them into the machine. (your instructor might do this for you.
Component TABLE
| Component & Conc. | amt. in a 50 µL μl reaction |
Final Conc. |
|---|---|---|
| Purified DNAase free Water |
26.25 μl | |
| 20mg/ml BSA | 2.5 μl | 1 μg/μL |
| 2.5 mM (each) dNTP mix | 4 μL | 200 μM (each) |
| 10x Buffer | 5.0 μl | 1x |
| 5 U/μL Ex Taq Polymerase | 0.25 μl | 1.25 U |
| 27F primer 3 μM |
5.0 μl | 300 pM |
| 1492R primer 3 μM |
5.0 μl | 300pM |
| template DNA | 2 μl | 10-30 ng |
PRIMERS:
- 27F AGA GTT TGA TC(C/A) TGG CTC AG Universal bacterial primer Lane et al. 1991
- 1492R (s) ACG G(C/T)T ACC TTG TTA CGA CTT Universal bacterial primer Lane et al. 1991
Hold the tubes on ice until your instructor tells you the thermal cycler is ready to be loaded. Wipe the outside of the tubes to remove all ice and water before placing them in the thermal cycler.
The thermal cycler program is, generally, similar for all PCR reactions, but the annealing temperature is dependent on the primer pair. When you design primers, the primer annealing temp. can be calculated based on the GC content and other factors. For 27F and 1492R, a range of 48-55 ˚C is ok, although higher temp. may lead to increased specificity that excludes some organisms' DNA from being amplified.
The length of the fragment you are amplifying determines the extension time. A general rule of thumb is to use an extension time of 1 kb per minute. Here, we amplify with primers designed for the 27th and 1492th positions in the 16S rRNA gene region based on E. coli 16S rRNA gene. Therefore our fragment is expected to be about 1.5kb long, so we will use an extension time of 1.5 minutes per cycle.
Thermal Cycler Program:
| Cycle Step | Temperature | Time | # of Cycles |
|---|---|---|---|
| Initial Denaturation | 95°C | 3 min. | 1 |
| Denaturation Annealing Extension |
95 ˚C 50 ˚C 72 ˚C |
30 sec 25-30 sec 2 min |
32 |
| Final Extension | 72 ˚C | 10 min | 1 |
| Hold | 4°C |
The PCR will run for 2+ hrs.
Part D: Agarose Gel Electrophoresis of PCR Product
1. Before adding 5 µL of your PCR product (PCRP) and loading dye to a well in the agarose gel please transfer 8 µL from your original PCR product tube to a new microfuge tube.
2. Label both of these tubes with site color coded tape and your site Code letter, initials, and PCRP. Leave room to add the concentration of DNA later. Keep both tubes on ice.
3. Use the original PCR tube to measure the post PCR DNA concentration using the nanodropper and to load the Agarose GEL. You will be adding 2 µL of EXOSAP to the 8 µL of PCR product in Part E of the protocol.
To see if you successfully amplified the 16S rRNA gene and not anything else, you will "run a gel" on your PCR products. To run a gel means that we will perform an electrophoretic separation of the DNA fragments in your PCR product, using 1/10 vol. of your PCR product applied to a 1% agarose gel stained with Sybr Safe DNA stain. Your instructor will photograph the gel, label it with your amplicon id from the template and post the gel photo to the Data folder in Resources in Sakai so you can evaluate your success at 16S rRNA gene amplification. You should see a single band of ~1.5kb indicating that the only dsDNA in your PCR product came from amplification of a ~1500 bp gene fragment. Can you explain how we know the size of our amplified gene fragment?
Your agarose gel is made of 1.0% agarose solution (w/v) in 1x TGE buffer (10x=0.25 Tris, 1.9M Glycine, 13mM EDTA) with SybrSafe™ stain.
DNA is uniformly negatively charged and will, therefore, move toward the positive electrode. The separation is determined by the size or mass of the molecule or fragments of DNA.

Procedure for Agarose Gel Electrophoresis of PCR products
Load 1/10 of the total volume of PCR product (1 µL minimum). In our case we should load 5 µL.
You will put the 5 µL of your PCR product as a spot on a small piece of parafilm and add a 5 µL amount of well mixed loading dye (0.25% XC, 30% glycerol, 0.1mg/ml RNAase). Mix by pipetting up and down before loading the "mix" into a lane of the 1% agarose gel (1% wt/vol in 1xTGE buffer with Sybr Safe DNA stain. Sybr Safe is a proprietary reagent from Invitrogen that cross-links a fluorescent compound to DNA and allows DNA to be visualized under UV light. It is prepared and used according to the manufacturer (Invitrogen) directions. Apply your samples to the wells that you have signed up for on the gel template. Be sure to leave the first two lanes and the last lane empty for the 100 bp ladder, the positive control and the negative water control.
Loading dye contains glycerol to keep our sample in the lane rather than floating away and will have one of 3 marker dyes (bromophenol blue, xylene cyanol, or
orange G) that facilitate estimation of DNA migration distance and optimization
of agarose gel run time. 1x TGE buffer is used in this electrophoretic separation (25 mM Tris, 0.19 M glycine, 1.3 mM EDTA. The gel will be run at 120V for approximately 30 minutes.
How will you judge a successful amplification? How many fragments and of what size do you expect to see?
Make sure you give back the rest of your isolate lysate and the rest of the PCR product, and the cleaned up PCR product tube (if performed in this lab) to your instructor to freeze after the gel is loaded. Both are now in identical looking microfuge tubes with volume being the only visible difference. Make sure it is clear which is the PCR product and which is the genomic DNA isolate.
Make sure eeverything is clearly labeled with your initials, lab section (Tues or Wed), soil sample identifier code letter, your unique code information.
Your instructor will photograph and label the gel according to the template you have filled out and post the results to the data folder in Resources in Sakai.
Part E: PCR Purification (Clean-Up)
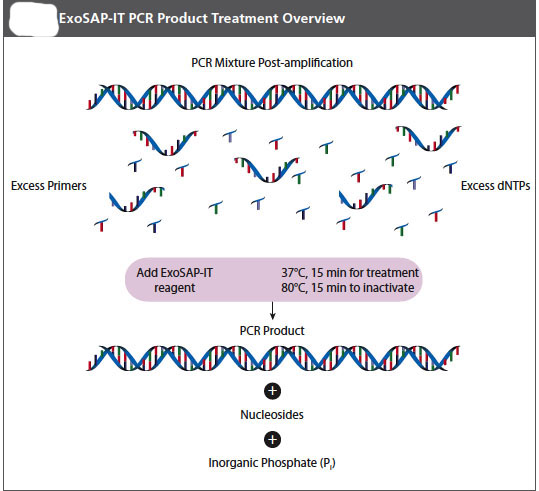
It is important that your pcr product be free of left over DNA polymerase, primers, and free nucleotides before a sequencing reaction is attempted on it. Your instructor will let you know if you will do this purification today after you have loaded part of your sample on the gel or if she will do it for you later, or, perhaps, we might have the sequencing facility do it for us. The important thing for you to know is that it must be done and that there are several methods commonly used to accomplish this purification. One of the simplest is an enzymatic teatment. We will use an Exosap-it® kit from USB.
USB ExoSap-iT PCR Product Cleanup (Product Number 78200)
Protocol
ExoSap-IT reagent treats PCR products ranging in size from less than 100 bp to over 20 kb with no sample loss. The clean-up reagent is proprietary, but it is based on Exonuclease I and Shrimp Alkaline Phosphatase. Recombinant (rSAP) is added directly to the PCR product to degrade primers and to dephosphorylate dNTPs that were not consumed in the reaction. Add EXSAP_IT reagent directly to the reaction products following PCR. Since ExoSAP-IT reagent is active in commonly used PCR buffers, no buffer exhange is required.
1. Remove ExoSAP-IT reagent from -20 ˚C freezer and keep it on ice throughout this procedure.
2. Mix 8μL of a post-PCR product with 2 μL of ExoSAP-IT reagent for a combined 10 μL reaction volume.
==
3. Incubate at 37 ˚C for 15 minutes to degrade remaining primers and nucleotides.
4. Incubate at 80 ˚C for 15 min. to inactivate ExoSAP-IT reagent.
5. The PCR product is now purified and ready for use in DNA sequencing or other primer extension applications.
6. Treated PCR products should be stored at -20 ˚C until required.
NOTE: ExoSAP-IT reagent must be stored in a non-frost free freezer!
Part F: 16S rRNA gene sequencing by Illumina™
Your instructors will send out your amplicons to a commercial sequencing facility for next-generation, high-throughput DNA sequencing of the 16S rRNA genes found in your soil DNA. We are using Illumina™ sequencing. The Illumina approach relies on attaching fragmented genomic DNA prepared in a sample library to a planar, optically transparent surface on a flow cell. These templates are sequenced using a fourcolor DNA sequencing-by-synthesis technology that employs reversible terminators with removable fluorescence. This highly parallel approach can generate close to 400 billion bases (Gigabases) with high accuracy, with 1.3 billion reads per flow cell run as paired-end 150 basepair reads. Labeled nucleotides are incorporated at each cycle and high sensitivity fluorescence detection is achieved using laser excitation and total internal reflection optics. Images are compiled and processed to produce base sequences for each DNA template. Applications are de novo sequencing where there is no reference available and resequencing, where short sequence reads are aligned against a reference. The genetic differences on the sequences are called using a specially developed data pipeline. We should get up to 15,000 reads per sample! Later in the semester when the data comes back you will have a workshop where we will see the processed data and learn how to evaluate it. The illustrations below come from the Dept. of Energy's Joint Genome Institute (DOE-JGI).



Media: DOE_JGI_Illumina_HiSeq_handout.pdf
Media: illumina_sequencing.pdf
You tube video showing an animated version of the process can be found at [http://www.youtube.com/watch?v=77r5p8IBwJk |http://www.youtube.com/watch?v=77r5p8IBwJk}
Characterization of Soil Bacteria: Maintaining Pure Cultures of Isolates
Continue to subculture your pure isolates to fresh NA plates each week (isolation streak a well isolated colony onto a fresh plate), in the next lab you will make a bacterial smear and do a Gram stain and start other tests to provide examples of co-operative and competitive community behavior among the members of your soil community.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
Graded assignment:
There is no graded assignment due next lab:) However, there is a graded assignment due at the beginning of Lab 7. If you want to get started on it the instructions can be found at Assignment: Molecular Techniques Theory and Use. Instructions: Assignment: Understanding Molecular Techniques
To Do Before the Next Lab:
You will need a fresh subculture of each of your isolates in Lab 6. It is important that they be in pure culture. Therefore, don't forget to come into the lab and continue sub-culturing until all the colonies on the plate appear identical. Approximately 24-48 hours before Lab 6 for fast growing organisms and 3-7 days (based on your experience) for slow growers. Set up a new subculture on a plate using a well isolated colony from each of your last streak plates. Please plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. The number of hours it takes from inoculation until a bacterial culture moves from log to stationary or death phase depends on its generation time, the concentration of the inoculum, and other factors.