BISC209/F13: Lab7
LAB 7: Diversity, Co-operation and Competition Among Selected Examples of Bacteria in a Soil Community: Assessing Bacterial Interactions, Functional Roles in the Nitrogen Cycle, and Misc. Characteristics
Confirmation of Gram stain results by Selective/Differential Media:
Did each of your isolates grow on PEA or EMB? What does that result mean about the isolate's cell wall composition? Do your Gram stain findings and PEA and EMB growth patterns agree? If not, how might you explain unexpected findings?
Complete the Motility Assessment & Analyze the Results
Soft Nutrient Agar Deep Medium
Use Nutrient Agar recipe but reduce agar content to 0.35% Bacteriological Agar. pH 7.6 at 25°C and pour into sterile glass tubes
MOTILITY ASSESSMENT
Look for radiating growth around the stab line of inoculation of each isolate in each of your soft agar deeps. Motility detection is possible due to the semisolid nature (low concentration of agar) of these soft agar deeps. Growth radiating out from the central stab inoculation line indicates that the test organism is motile. First hold an E. coli positive control tube up to the light to see an example of radiating growth. Growth appears cloudier than the medium. Compare your positive control to an uninoculated tube and to a negative control culture of a non-motile organism. Non-motile bacteria exhibit growth in a tighter, defined line limited to where the organism was inoculated. In contrast, motile organisms exhibit detectable growth radiating away from the stab inoculation line towards the periphery. Strictly aerobic organisms may show more growth radiating down from the surface of the medium compared to the growth deep in the tube. Consult with your instructor if you are having a hard time deciding whether or not your isolates are motile. Why might it be useful for some soil community members to be motile?
If you have time, you can try to confirm a positive preliminary motility test by doing a hanging drop motility wet mount or a flagella stain. See the Protocols section in the wiki on Motility Tests for directions on performing confirmation tests.
Continue Antibiotic Production test started last week
Week 2
Need fresh control cultures of Eschericia coli (Gram negative), Staphylococcus epidermidis (Gram positive) and Micrococcus luteus (Gram positive) grown in nutrient broth to the same turbidity standard used last week for isolate cultures. Use the MacFarland Standard to make sure that these cultures are of the same turbidity as your isolates were when you applied them to the NA plates last week. If not, dilute a little of the stock control cultures (in separate sterile tubes)to appropriate turbidity with nutrient broth.
PROTOCOL
Use the cultures of your isolates set up last week on NA.
Use a sterile swab to aseptically apply parallel lines of inoculation of each of the control broth cultures of : E. coli, Micrococcus, and S. epidermidis as shown in the illustration below. Use a different sterile swab for each culture. These parallel inoculation lines should be made perpendicular to the putative antibiotic producer's (your isolate's) colony growth. (See the illustration.) Be careful not to touch the putative antibiotic producer's growth with the control cultures, but come as close as you can. Make a template in your lab notebook and label the plate to indicate where each control culture is streaked. Incubate these culture plates at RT in a place that where your instructor can monitor their development. One person/lab should also set up viability controls by swabbing each the three broth cultures onto separate areas of another NA plate. If you see growth of each of the controls next week, we will be sure that any inhibition of growth is due to sensitivity to a diffused antibiotic rather than lack of growth occurring because one or more of the control broth cultures you used today lacked enough viable cells to form colonies on your test plate.

Antagonistic and Mutualistic Interactions Analysis
*NOTE: You must remember to set up fresh nutrient broth cultures for your isolates 1-3 days before lab to do this test!
The microbial community living in soil is a complex one with many different microorganisms. As is true of any environment, these microbes interact with each other - both functionally and physically. Do selected bacteria from your community help each other or harm each other while trying to find a niche in your soil community? Today, you will try to answer that question by testing your cultured isolates for examples of mutualism or antagonism (co-operation or competition)by culturing them in controlled communities. Some of these bacteria may prevent the growth of others through the production of chemical inhibitors; others might promote the growth of their neighbors by producing metabolites that are needed. We are going to look for both positive and negative interactions.
PREPARING THE ISOLATES:
You will inoculate 50 µl of log phase (young culture) isolate grown in fresh nutrient broth into the assigned well(s). Once again try to control for similar numbers of organisms in your inoculum using the 0.5 McFarland standard, diluting the culture or adding more organisms as needed.
Interaction Assay Set Up
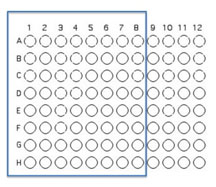
You will use 64 of the wells on a 96 well plate for this assay. Each pair will use 8 unique isolates (4 from each student) to test for interactions. Use the Excel template provided Media:template.xls to record the identifying codes of the organisms that will be inoculated into each well as described and illustrated below.
FOLLOW THE TEMPLATE CAREFULLY!!!!!! It is easy to get this inoculation messed up, but don't!

Transfer 50 μL of each of 8 unique isolates to be tested into the illustrated row of wells (A1 is Isolate 1, A2 is Isolate #2 etc through A8)
Beginning with Isolate #2, inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color).
Add 100 μL of nutrient broth to each of the wells containing your isolates (row wells A1-A8 and column wells B1-H1)
Gently move the 96 well plate in a circular motion to mix.

Transfer 10 μl of the contents of the 7 wells - A2 (containing isolate #2 etc) through A8 to the empty wells in each column as indicated by the yellow arrows. You will need to remove the first tip from a multichannel pipette. If you are using the multichannel pipette, be sure that you work slowly and check that each pipette tip is evenly filled. You may need to tighten the tips by hand, if so be sure to only touch the part of the tip that sits on the multichannel pipette, you wouldn't want to contaminate your wells with human organsisms!
Transfer 10 μl of the contents of wells A1 (containing isolate #1 etc) through H1 to each well in the row as indicated by the red arrows.
Again gently mix the contents of the well by moving the plate in gentle circles.
NEXT, we will inoculate a square (NUNC) tray containing nutrient agar medium with about 5 μl of the contents of the wells we just prepared. For this step we will use either a tool called a "frogger" or a multichannel micropipette. If using the frogger, dip the tips into 96 wells to attract a drop of inoculum onto the end of each steel tip and then touch the those tips to the surface of the sterile NA square NUNC plate. Do not break the surface of the agar but make sure your pressure is even so every steel tip has touched the agar surface and deposited the same inoculum. Be sure to disinfect the frogger by dipping it into a series of disinfectant and rinse solutions that you will find at the cleaning station prepared for you .
If the frogger is not available, use an 8 channel multichannel pipet set to 5µl and remove 5μL of culture from each well of your culture dish and deposit all of it onto an area of the NA square agar NUNC plate that is in the same location as in the 96 well culture dish. Again, be sure the tips are on tightly before loading the pipet. Repeat this procedure, with new tips, for each ROW of 8 wells until you have completed depositing the full array in the same orientation as the 48 wells.
7. Wait for your inoculated spots to dry, seal or cover the NUNC square tray, and incubate at Room Temp. The 96 well plate was used only to mix the cultures so you can discard this in the appropriate manner. In 24 hours we will need to transfer a small sample from each colony for analysis by MaldiTof Spectroscopy. If so, your instructor will provide more information. You will check on your assay and note any differences in the appearance of the colony growth of each isolate, alone vs mixed next lab.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
Graded Assignment:
Directions for this assignment are found at: Assignment 7.
To Do by the Next Lab:
Continue a fresh subculture of your pure isolates today and prepare a fresh isolation streak subculture 24 hours prior to lab 8 so we can begin freezing your isolates.