BISC209/S11: Lab3
Whole Community Microbial Analyses: Enumeration & Function
Because each type of bacteria in a soil community fills a unique niche and may play a slightly different role in nutrient cycling or soil structure, it is important to identify group members and to study them individually, if that's possible. However, it is equally important to study the cumulative functionality of the community.
In our investigation of bacterial soil community diversity through culture independent analysis of 16s rRNA relatedness, we hope to establish some surprising evolutionary lineages since so few of the soil bacteria have yet been studied or identified. In contrast, the culture-dependent work at the soil microbial community level that we will set up today will have the goal of studying functional rather than phylogenetic diversity, because community function is at least as important as phylogenic diversity to a soil community. In addition to phylogenetic diversity, soil microorganisms demonstrate extreme diversity in their abilities to alter raw materials into useable nutrients. A soil community can work co-operatively to share the “work” of processing available raw materials into a form that members can use. Bacterial communities can be thought of as functional units comprising the sum of the metabolic properties of its members. Functional diversity in substrate utilization for energy metabolism is of great importance, particularly if one is concerned about the long-term health of a habitat or soil community.
One of the reasons bacteria can exist in large numbers in a small area is because they do not necessarily compete for the same raw materials. Not all microbes synthesize the same metabolic enzymes; those enzymes control which nutrients a soil community member can use or process. Even closely related species in the same genus can show very different enzyme profiles, something that is not obvious from a phylogenetic tree of evolutionary relatedness. Because soil bacteria and fungi are the microorganisms largely responsible for the turnover of plant material, changes in the numbers of these microorganisms can be related to changes in the organic matter content of soil.
The soil bacterial community is not only interdependent on its bacterial members, but it interacts extensively and crucially with eukaryotic members as well, particularly in the rhizosphere: the soil that is in contact with plant roots. Rhizosphere microbes promote plant growth and vice versa. Today you will use a community level culture-dependent method of quantifying bacteria performing those important functional roles for their soil community.
To begin your work assessing microbial diversity and abundance at the community level, you will need to acquire another soil sample.
Make another Soil Extract and Serial Dilution
You will prepare another soil extract today from a new soil sample so that you can set up some tests for the prevalence of specific exoenzymes that have an important impact on the availability of nutrients for the soil community. You will start some measurements of metabolite cycling and utilization. We will assess, specifically, at the community level, how well your soil microbes interact co-operatively to use a variety of carbon sources, and to process or digest phosphates, cellulose, and starch.
Our goal is to obtain quantitative evidence for the abundance and richness of the microbial soil community--- more specifically, to answer the following questions: how well does this soil microbial community use a variety of carbon sources for its anabolic needs, process phosphates, and how well does it perform important catabolic processes such as cellulose and starch degradation?
Soil Extract Preparation:(one extract and dilution series for each worksite)
- Using a freshly collected and sieved soil sample collected prior to lab process the soil as you did in lab 1. Weigh 1 gram of sieved soil using the top loading balance and add it to 100 mL of sterile water which you will find premeasured for you in a sterile flask on your bench.
- Swirl to mix--- don't use the magnetic stirrer yet.
- Pour this soil suspension into a clean blender jar. Use gloves when you place the cap on the jar, first making sure that the rubber gasket is properly positioned and the seal at the bottom is tight (otherwise it will leak!).
- Blend for 3 pulses of 10 seconds on and 10 seconds off.
- Pour all of the suspension back into the flask and add a sterile magnetic stir bar (on your bench).
- Place the flask on a magnetic stirrer at medium speed and mix for at least 15 min.
- Stop the stirring and let the soil settle until the larger particulate matter settles to the bottom. Not all visible particles need to settle, just the big stuff.
- Pour off about half of the supernatant (avoiding transferring the settled particles) into a new, labeled, sterile 50 ml conical tube. In making this extract, you have created a 10-2 or 1/100 dilution. This extract can also be described as a 1% (wt/vol) solution since there is 1 gram/100ml.
Serial dilutions: (one dilution series per soil extract).
You and your partners will prepare a series of dilutions to use in the community physiology profiling analyses that we will set up today.
- 4 large (13 X 100), sterile tubes with caps that can hold a volume of 10 ml and not spill it when vortexed
- 4 1ml sterile, disposable pipets and a blue pipet pump
- 1 10ml sterile, disposable pipet and a green pipet pump
- P1000 micropipet and sterile tips
- Bottle of sterile water
1. Label four large sterile tubes 10-3, 10-4, 10-5,and 10-6.
2. Pipet 9 ml of sterile water into each tube using a 10ml sterile, disposable serologic pipet. (You may use the same sterile 10 ml pipet for all of the tubes if asepsis is maintained.)
3. Using a different sterile, disposable 1ml serologic pipet for each transfer, transfer 1ml of the 1% soil extract (1:100 dilution made of 1gram of soil) to the tube labeled 10-3; mix well by vortexing.
4. Using a new pipet, transfer 1ml of the 10-3 dilution to the tube labeled 10-4. Mix well. (Mixing 1ml of the 10-3 dilution with 9ml of sterile water makes a 10-4 dilution. )
5. Continue to transfer 1ml aliquots (after mixing well) from each dilution to the next dilution tube of water until you have carried the dilution to 10-6.
- Community Carbon Sources Utilization
- Exoenzyme Assays for Starch digestion, Cellulose digestion, and Phosphate solubilization.
Enumeration of Whole Community Soil Microorganisms
After preparing the soil extract, please transfer 1.0 ml of the soil extract (10-2) to a new microfuge tube. Label the tube with your work-site letter using a piece of your team color tape. Make sure all the labeling is legible!
Give this tube to your instructor. The course instructors will stain the microbes with a fluorescent DNA stain (DAPI), transfer the microorganisms to a filter, and photograph the microbes using fluorescence microscopy (as described below). These photomicrographs will be made available on Sakai. Make sure you view and perform the calculations described below to assess the total microbial concentration in this culture-independent enumeration of your soil community's microorganisms. Compare this number to your calculation of CFUs/gram of wet soil (a culture-dependent assessment). Do you have evidence that culturing microorganisms is a poor way to determine who's there?
DAPI DNA stain protocol:
NOTE: The staining and imaging protocol will be performed by your lab instructor and an image posted for enumeration. The protocol below is for reference only. YOU WILL NOT PERFORM THIS IN LAB!
DAPI aqueous solution Stock conc. is 1mg/ml : 4'-6-Diamidino-2-phenylindole (DAPI) is known to form fluorescent complexes with natural double-stranded DNA.
- Add 1 μL DAPI stock stain to 1mL of diluted soil extract so the effective concentration of DAPI is 1μg/mL.
- Incubate at 4°C for 20 min.
- Set up a vacuum flask and filter apparatus (125 ml side-arm flask, Borosilicate base and fritted glass filter support with rubber stopper)
- Carefully place a 0.2μM glass fiber filter (Isopore membrane GTTP02500) onto the fritted glass filter support.
- Add the borosilicate glass funnel onto the base and clamp the two section together using an anodized aluminum spring clamp.
- Rinse the filter once with 1 mL sterile deionized water using a vacuum pump to provide 178mm Hg of force. Wait until all the water is removed.
- Turn off the vacuum, carefully break the seal to release residual vacuum and carefully transfer the 1mL of DAPI stained extract (made in step 1) onto the 0.2μM glass fiber filter.
- Turn the vacuum on and wait until all the solution is filtered.
- Dissassmeble the funnel and gently break the vacuum by loosening the rubber stopper.
- Use forceps to carefully remove the wet filter and place it on a microscope slide.
- Add a coverslip and 1 drop of Fluoro-Gel with Tris buffer (Electron Microscopy Sciences Cat #17985).
- Use the Nikon Eclipse 80i flourescent in L318C to view and photograph the DNA at 1000x magnification.
- Use the Nikon NIS-Elements Imaging software, to photograph the a field of view in white light, save this image, then do the same using the UV light at 350nm excitation wavelength (filter #1).
- Count the unique spots of blue fluorescence; each indicates a soil microbe's genetic material (chromosome or nucleus). It is assumed that the bacteria are arranged in a Poisson distribution. For the most accurate counts, 20 fields or 400 bacteria should be enumerated to determine the number of bacteria per ml.
CALCULATIONS:
You will use a photomicrograph of a representative field of view posted by your instructor to count the fluorescent "dots" (microbes) and calculate the number or organisms in 1 ml of soil extract.
The area of each field of view at 1000X using the Fluorescent scope is 10487μmeters2. The diameter of the filterable section of the borosilicate apparatus is 17 mm. So the area is 2.3 X 105μM2. Multiply the number you counted on the photomicrograph by a factor of 21.6 (2.3 X 105μm2 divided by 1.0487 x 104μmeters2) to determine the number of organisms found in 1mL of filtrate of extract. Then correct for the 1:100 dilution factor for the number of organisms in 1 gram of wet soil.
Adapted from Schallenberg, M., Jacob, F. and Joseph B. R. (1989) Solutions to Problems in Enumerating Sediment Bacteria by direct counts. Applied & Environmental Microbiology. p. 1214-1219.
Community Physiological Profile of Starch Digesters, Cellulolytic Bacteria, and Phosphate Solubilizing Bacteria: Background
Degradation of complex carbon containing materials such as leaves and other plant matter provides an important source of a wide range of organic molecules to heterotrophic members of a soil community. Cellulose and starch are two complex carbohydrates that can be important sources of carbon, if broken down to a simpler, useable form. Many, but not all, soil bacteria contain the enzymes necessary to play this important role. It is not surprising that bacteria and other microbes are able to digest molecules associated with plants since plant roots are always present in the soil and plant parts become part of the soil litter that adds organic matter.
Starch Hydrolysis: Starch is a common plant storage compound. It is a complex polysaccharide carbohydrate of sugar (glucose) molecules joined by glycosidic bonds. If the hydrolytic exoenzyme amylase is produced, the starch in the medium will be degraded. Microbes capable of degrading starch extracellularly to molecules small enough to be taken up by the cell can use the mono or disaccharide products of starch digestion to produce energy (ATP).
Cellulolytic Activity: Cellulose is an organic compound with the chemical formula (C6H10O5)n. It is a polysaccharide consisting of several hundred to over ten thousand linear, linked β(1,4) D-glucose units. Cellulose is a primary structural component of the cell wall of green plants, many forms of algae and some other types of organisms. Some species of bacteria secrete cellulose to form biofilms. Cellulose is the most common organic compound on Earth comprising 40 to 60% of plant residues. Some animals can digest cellulose (e.g. cows, termites) with the help of symbiotic microorganisms that live in their guts. Humans can digest cellulose to some extent, but cellulose consumed by humans as food is largely undigested and acts as a hydrophilic bulking agent for feces ("dietary fiber"). Soil microorganisms capable of catabolizing cellulosic material contribute to the carbon cycle and, ultimately, contribute to the release of CO2to the atmosphere--- making it available for re-uptake by plants.
Cellulolysis is the process of hydrolytic break down of cellulose into smaller polysaccharides called cellodextrins or complete break down into glucose units. Because cellulose has a somewhat complex structure, Cellulolysis is relatively difficult compared to the breakdown of other polysaccharides. The enzymes used to cleave the glycosidic linkage in cellulose are glycoside hydrolases. They include endo-acting cellulases and exo-acting glucosidases. Such enzymes are usually secreted as part of multienzyme complexes. Soil bacteria and symbiotic anaerobic bacteria (like the Cellulomonas group of bacteria) are among those known to produce these enzymes. Some termites contain flagellate protozoa in their hind guts that produce cellulases while other termites rely on gut bacteria. Fungi are also able to break down cellulose.
Phosphate Solubilizing Activity: Phosphorus is one of the major nutrients that limits plant growth. It is important in physiological activities such as photosynthesis, root development, cell division, and for efficient use of carbon substrates. Most phosphorus is found in an insoluble form; thus, the conversion of insoluble phosphorus into soluble forms that can be absorbed by plants is an important interaction between plants and phosphate solubilizing bacteria.
Because soil is, in some ways, a non-renewable resource, the activity of the microbial population in maintaining soil quality is of paramount importance and a diverse and rich population of phosphate solubilizing bacteria play a crucial role in the improvement and repair of soil health destroyed by years of environmental abuse.
Community EXOENZYME Profiling:
Activities:
Total culturable bacterial enumeration
Starch hydrolysis assay
Cellulose digestion assay
Phosphate Solubilizing assay
Protocol for all exoenzyme assays:
Refer to your standard plate count calculations (Lab 2). You will use the dilution that gave you 30-300 CFUs when you evaluated your plate count in Lab2 AND one dilution higher and one dilution lower. Use those 3 dilutions to inoculate the media described below. Using three dilutions will ensure that at least one plate of each medium will have colonies of the appropriate density to be accurately counted and evaluated next week.
1. Nutrient Agar (NA) General Purpose Medium is used to determine comparative number of total culturable bacteria: Using your P200 micropipet and sterile tips, dispense 100µl of the appropriate soil extract dilution onto a pre-labeled Nutrient agar plate. Use a sterile, disposable spreader to evenly distribute the diluted soil extract all over the culture plate. Repeat for the other two appropriate dilutions. 24-72 hours later refrigerate your plates to examine next lab.
Nutrient Agar General Purpose Medium:
0.3% Beef extract, 0.5% Peptone, 1.5% Agar at pH 6.6- 7.0 at 25°C.
2. Starch Medium is used to determine the % of amalyase producing, starch digesting culturable microbes when compared to the total number counted in NA: Using your P200 micropipet and sterile tips, dispense 100µl of the one of the appropriate soil extract dilutions onto a pre-labeled starch agar plate. Use a sterile, disposable spreader to evenly distribute the diluted soil extract all over the culture plate. Repeat for the other two appropriate dilutions. 24-72 hours later refrigerate your plates to assay next lab. Next lab flood the plate with Grams Iodine, look for colonies with a clear halo indicating the starch had been digested. Count the number of colonies on the plate and the number that are able to digest starch (clear zone around the colony) and calculate the % of cellulytic microbes.
Starch medium :
0.3% (wt/vol) soluble starch in Nutrient Agar
Reference: Beishir, Lois. 1996. Microbiology in Practice 6th ed. HarperCollins Publishers Inc. New York. Module 33: 301-306.
3. Cellulose Medium is used to determine the % of cellulolytic microbes (those producing cellulase) when comparted to the total number counted in NA : Using your P200 micropipet and sterile tips, dispense 100µl of the one of the appropriate soil extract dilutions onto a pre-labeled cellulose congo red agar plate. Use a sterile, disposable spreader to evenly distribute the diluted soil extract all over the culture plate. Repeat for the other two appropriate dilutions. 24-72 hours later refrigerate your plates to assay next lab. The ashed, acid-washed cellulose and the Congo red in the plates we will use in our cellulase detecting assay will allow enumeration of cellulose-utilizing bacteria in soil. Next lab you will look for distinct clear zones around microbial colonies. The red color of the medium provides increased contrast between the cellulose containing medium and the halo or clear zone that indicates that cellulase has diffused out of bacterial producers and degraded the cellulose in the immediate vicinity. count the number of colonies on the plate and the number that are able to digest cellulose (clear zone around the colony) and calculate the % of cellulytic microbes.
Cellulose Congo Red Agar:
0.05% K2HPO4; 0.025% MgSo4; 0.188% ashed, acid washed cellulose powder; 0.02% Congo red, 0.5% Noble Agar, 0.2% gelatin, 10%(vol/vol) sterile soil extract. (Soil extract prepared as follows:105 g of air-dried sieved soil and 660 ml of deionized water are placed in a 1 litre bottle and autoclaved once at 15 psi for 15 minutes, then again after 24 hours. The contents of the bottle are left to settle for at least a week and then the supernatant is decanted and filtered. The final pH should be 7.0 - 8.0.)
Reference: Hendricks, C.W., Doyle, J.D., Hugley, B. (1995) A new solid medium for enumerating cellulose-utilizing bacteria in soil. Applied and Environmental Microbiology 61, 2016-2010.
4. Phosphate Medium (Pidovskaya medium [PVK]) is used to determine the % phosphate solubilizing microbes (those producing phosphatases) in a soil community: Pipet 100µl of the 3 selected soil extract dilutions onto separated labeled Pidovskaya medium plates. Use a sterile, disposable spreader to evenly distribute the diluted soil extract all over the culture plate. Repeat for the other two appropriate dilutions. 24-72 hours later refrigerate your plates to assay next lab. A halo will be present if the organism was able to digest the phosphate producing an acid byproduct. Count the total number of colonies and the number with halos. What % of the community growing on this plate are able to digest phosphate?
Pidovskaya Medium Modified (Nautiyal, 1999)
1% glucose; 0.5% Calcium Phosphate [Ca3(PO4)2; 1% MgCl2.6H2O; 0.25% Magnesium Sulfate (MgSO4.7H20);0.2% (NH4)2SO4; 0.25% KCL; 0.0025% BromoPhenol Blue; 1.5-2% agar ; distillled water 1000 ml.
References: Pikovskaya, R.I. 1948. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologia 17, 362-370>
Pranjal Baruah (2007)Isolation of phosphate solubilizing bacteria from soil and its activity. Available at: Biotechindia.files.wordpress.com/2007/12/isolation.pdf.
5. Incubate all the cultures at room temp for 48-72 hours in a plate holder. These plates should be moved to the cold room when visible colonies have formed and matured and before they overgrow the culture plates.
6. In Lab 4 you will examine the plates for processing of the relevant nutrient in each culture medium. You will count the number of individual colonies that show a clear zone (halo) around the colony (using the plate with 30-300 total colonies).
Community Carbon Source Utilization Profiling:
Carbon Source Utilization
One type of metabolic diversity that we will assess in our investigation is physiological diversity in carbon source utilization.
You have learned in other courses about the importance of carbon fixation by autotrophic photosynthetic plants. The inability to make carbon-carbon bonds and, therefore, to utilize carbon dioxide as a carbon source is problematic for heterotrophic species including humans and all other animals. Fortunately there are bacteria that, like plants, are autotrophic and photosynthetic, although many others are heterotrophic, like us. Unlike us, however, bacteria are extremely diverse in the types of carbon sources they can use metabolically. Soil bacterial communities both compete and co-operate in utilization of available sources of essential, useable carbon. The health and longevity of the community is dependent on a continuous supply of useable carbon for all its members. Your investigation on community carbon source profiling will attempt to quantify some of that co-operation and competition.
Carbon source patterns using BIOLOG™ Community Level Physiological Profiling (CLPP)
Observing patterns of substrate utilization can provide evidence of functional diversity of a microbial community. Additionally, understanding how metabolic substrates can be used in soil communities can help us understand the stability of an ecosystem. Carbon sources are crucial anabolic raw materials for heterotrophic microbial growth. Microbes vary enormously in their ability to make use of carbon in different forms. Using direct inoculation of soil community samples into a variety of carbon substrates will allow us to study and measure potential community carbon source utilization and provide us with evidence for our hypothesis that soil community diversity allows co-operative as well as competitive interactions.
In community level physiological profiling (CLPP) the metabolic properties of individual bacteria in the community contribute to the total metabolic capacity of the community. Mixed environmental samples are inoculated directly into the single carbon source wells of microtiter plates followed by spectrometric quantification of growth. If one or more microbes in the community can use a particular carbon substrate, the metabolism of that carbon source is accompanied by a capture of electrons from water-soluble colorless Tetrazolium salts (WTS) to become reduced purple formazans. WST-1 and in particular WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium), are advantageous are reduced outside of the cells. They combine with PMS electron mediator to yield a water-soluble purple product called formazan that can be measured spectrophotometrically at 590nm. The color development is is additive and directly proportional to the metabolism of each carbon source so the development of forazan can be followed over time. The intensity of purple color as a pattern in the wells can used to determine average metabolic response (AMR) and community metabolic diversity (CMD). For these measurements to be meaningful, it is important to control for number of microbes, incubation time, and other microenvironmental factors as well as the requirement for saturating substrate and indicator concentrations.

Scheme showing the reduction of MTT to formazan. Image created by Jenpen 21 September 2006
Source http://en.wikipedia.org/wiki/File:Mttscheme.png . Public domain use per Wikipedia Commons.
Colorless (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) is reduced to purple formazan
The BIOLOG-ECO™ 96 well plates we will use contain 3 replicates of 31 carbon sources and three water control wells. Most of these substrates are commonly associated with plant root exudates and, thus, are likely to be available to your soil community. The method for community carbon source profiling that we are using is simple and rapid, but its interpretation must be carefully evaluated, recognizing that the methodology is imperfect. The following studies discuss issues and limitations of CLPP (community level physiological profiles) analysis.
These or similar references are provided in the lab Sakai site as .pdf files.
References and Resources: Biolog Carbon Source
• Garland, J.L., Mills, A.L. (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level-sole-carbon-source-utilization. Appl Environ Microbiol 57, 2351–2359.
• Garland, J.L. (1997) Analysis and interpretation of community-level physiological profiles in microbialecology. FEMS Microbiol Ecol 24, 289–300.
• Preston-Mafham, J., Boddy, l., Randerson, P.F. (2002) Analysis of microbial community functional diversity using sole carbon source utilization profiles-a critique. FEMS Microbiology Ecology. 42, 1-14.
PROTOCOL:
Carbon source patterns using BIOLOG™ Community Level Physiological Profiling (CLPP)
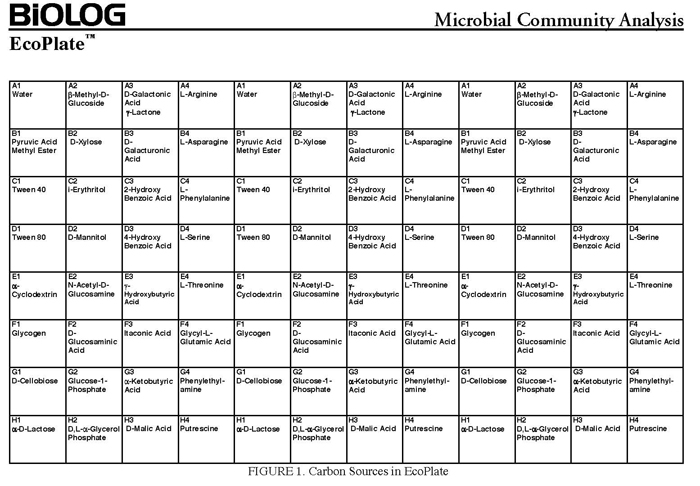
- 10mM phosphate buffer (sterile, pH 7)
- P200 and P1000 micropipets with sterile tips
- Multichannel pipet (set to deliver 100 µl) and sterile tips
- BIOLOG EcoPlate™
- sterile plastic multichannel reservoir
- Wear gloves throughout the entire protocol
- Do not cross contaminate your samples or the solutions
- Keep your work area clean: freshly disinfect your bench top before beginning
- Do not use a vortex at any point in this protocol unless it is specified that you should do so.
1. Use sterile serological pipets to make 12 mls of a 10-4 dilution (equivalent to 106cfu/ml). You will accomplish this by pipetting 10.8 mL of 10mM phosphate buffer into a large sterile tube and adding 1.2 mL from your 10-3 soil extract serial dilution tube. (Note: You need 12 ml of diluted soil extract to inoculate all the wells of a BIOLOG™ECO plate (10 ml for the plate and 2 ml extra.) Our goal is to end up with 105 soil microbes in each well (in a 100μL volume).
2. If you will be using a multichannel micropipet, pour this 12ml of diluted soil extract (106 cfu/ml) into a sterile reservoir (a petri dish or a v-shaped plastic reservoir). If the multichannel pipet is unavailable, forget pouring the soil extract dilution into a reservoie and use your P200 to dispense individual 100 μL aliquots directly from the dilution tube into each well of the BioLog plate.
4. Be careful to preserve the BIOLOG Eco™ plate's and its cover's sterility (eg. don't place it face down on your bench). Remove the cover and transfer 100µl of the soil extract dilution into each each well of the 96 well BIOLOG plate. Check visually the consistency of the amount of diluted extract in the micropipet tips after you have drawn up your aliquots to determine that you have no bubbles and that the quantity to be dispensed is the same. If the pipet tips appear unevenly filled or you have bubbles, do not dispense the inoculum into the wells! Start over. If you are unfamiliar with the use of multichannel pipets, ask your instructor to observe your technique.
5. Replace the cover of the plate and label one side of the cover. DO NOT LABEL ON the top or bottom to avoid interference in the light passage during spectrophotometric readings. Use a piece of your team color tape and include your initials, lab section, date, and soil sample code.
6. Take a time 0 reading at A590nm using the Spectramax340PC384 plate reader as described in the next section of these instructions.
7. After each reading, place your covered plate in a plastic container with moist paper towels and incubate it at room temp (~22C) in the location assigned for your lab section.
MEASURING MICROBIAL GROWTH AS A590nm
Within each well is a carbon source and a tetrazolium dye. Use of a carbon source by the microbes in the community is indicated by a redox indicator that measures metabolic reactions as development of purple colored reduction product . The intensity of color change is monitored in each of the wells by taking spectrophotometer readings once a day at A590nm. You and your partners should figure out a schedule to divide up the work of collecting these data until a peak absorbance is reached on more than 2 consecutive readings, this will likely require daily readings for 10-12 days. You must not miss more than 1 consecutive day. Make sure that you take a photo of your plate against a white background on the final day of measurement.
Time 0: Use the SpectraMax 340PC 384 spectrophotometer (with a microplate reader) found in the Microbiology lab (L302). You will measure absorbance as A590nm. Follow the directions for setting up the instrument and software and for exporting the data to Excel spreadsheets. You will find a copy of these directions beside the SpectraMax340PC and in the protocol section at BISC209/S11: SpectraMax 340PC instructions. The directions are also found in the next section:
Using the SpectraMax 340PC384 Microplate Spectrophotometer and SoftMax Pro version 4.0™ software
Spectramax 340PC :
Molecular Devices Spectramax 340PC instructions
Turn on the Spectramax 340PC spectrophotometer using the switch located next to the plug in the back on the right hand side (as you face the spec). The drawer will open. The drawer may, or may not, close on its own. If it doesn't, close it by pressing, once, the DRAWER button found on the Spectramax control panel (pictured in purple on the instrument photo below). It is important to keep the drawer closed as much as possible to prevent dust from entering the spectrophotometer. The machine will automatically start a required calibration. Allow up to 10 minutes for the Spectamax340PC to warm up after the calibration and BEFORE you attempt to insert your plate.

The computer should be on. (If not, turn it on using the on/off switch on the processor on the floor).
After the spectrophotometer finishes its start-up calibration, the drawer will open. If it doesn’t close on its own, close it by pushing ONCE the “drawer” button on the face of the spectrophotometer.
When the warm up period is over, double click on the SOFTmaxPRO4.0 shortcut on the desktop of the computer. An “untitled” document will open on the computer screen called Default Tutorial 1.
The draw may open again, or you can open it manually by pushing the DRAWER button ONCE. (Drawer button is found on the Spectramax control panel--as pictured in purple on the instrument photo above.)
Position your 96 well plate into the tray drawer so that it fits securely in the holder and the well A1 is to the top left. Close it using the DRAWER button .

Double Click the setup button located beside Experiment 1: ![]() Plate #1 . set up
A new window will open called Instrument Settings. The icon ENDPOINT will be active.
Plate #1 . set up
A new window will open called Instrument Settings. The icon ENDPOINT will be active.

Set the correct wavelength (590nm for our Biolog®: carbon source plates). Click OK
You will see a table representing a 96 well plate under EXPERIMENT #1. The spec is ready to read all the wells in a 96 well plate.
In the upper toolbar menu at the top of the screen click READ.
Troubleshooting: If the READ button is gray and won’t let you start the read, check for a red X over the PORT SERIES icon to the left of the READ button. It indicates NO PORT SELECTED. If so, click on this icon and select series port: COM 2 from the drop down menu. Click OK. (The drawer may open and you will need to close it).
You will hear the spectrophotometer slit lamp passing over the plate to "read" it, meaning that it is measuring absorbance at the nm wavelength best for detecting purple colored pigments (590nm). The purple color formation is a function of the total redox reactions that occurred in the well as the microbes use each carbon source metabolicaly and change the colorless form of the dye, 5-cyano-2,3,-ditolyl tetrazolium chloride (CTC), to a purple- colored product.
The drawer will open when the readings are completed. Remove your plate and CLOSE the drawer using the DRAWER button on the face of the spectrophotometer. It is important to keep the drawer closed as much as possible to prevent dust from entering the internal parts of the machine.
A new screen will appear with your recorded data in a 96 well template.
As a safety precaution, immediately export these data on the computer desktop to the BISC folder for your lab section. Use File: Import/Export: Export . Be sure to label the file with your soil sample code.
Now save the data in EXCEL: HIGHLIGHT and COPY all the data in the 96 well template.
Open a new Excel spreadsheet by clicking Start: Programs: Excel
Paste the data from the 96 well template: EDIT: PASTE SPECIAL: TEXT into the Excel spreadsheet. This is a very important step because it aligns the cells properly.
Label the wells in the spreadsheet A-H and 1-12.
Add identifying information to the worksheet title including: Biolog/590nm: Date, Soil Sample ID code: Group color and Lab Day.
Click Save As and rename the file with your group’s soil sample code and lab day.
Close the Spectromax software. DON'T SAVE! (You have already saved and exported adequately.)
Because the instrument computer is not networked, you will have to Save the excel spreadsheet to a FLASH DRIVE. If you don't have your own, there should be one in the USB port in the front of the computer processor (found on the floor under and to the left of the spectrophotometer). This one is provided for all BISC209 students. If the flash drive is missing, look to see if the last user left it connected to the Mac computer on the Instructor’s bench. If it is not there, you will have to send a message to the class through Forums in Sakai to see if someone took it home by mistake and email your instructor.
If you have a flash drive available, open Excel and use the FILE drop down menu to SAVE AS: (provide an identifying title with your soil sample code letter and your team color and lab day) and SAVE TO REMOVABLE DISK (E), BISC 209-2011 folder: Lab section (TUES OR WED) folder. Make sure your data is also saved to the instructor's computer hard drive so that you can retrieve it later. If you have your own Flash Drive, use that.
To remove a Flash Drive from a PC: Close the Excel document, you created, and click on the icon with a green arrow in the far right of the toolbar. A new screen will open “Unplug or eject hardware”. Click stop. In the “stop a hardware device” screen, highlight “generic volume device : E”. Click OK. The final screen tells you that it is safe to remove device. Click OK and Remove the Flash Drive from the computer.
Now send your data to yourself and to your partners through FIRST CLASS: Take the Flash drive over to a networked computer (the instructor computer in the lab is networked).
Go over to the Instructor’s Mac computer at the front of the lab and insert the flashdrive. You will see an image appear on the desktop of the Mac. Click to open it and find your data. Open FIRST CLASS and send the data to yourself, your partners and also place it in the folder for your worksite (Tues lab A,B,C) or Wed lab (D,E,F) in the RESOURCE folder in SAKAI. Please put this "raw" data into DAILY RAW DATA folder. When you have sent your data, drag the FLASHDRIVE icon to the TRASH and wait for it to disappear from the desktop. REMOVE THE FLASHDRIVE AND RETURN IT TO THE USB PORT ON THE SPECTROMAX PROCESSOR WHERE YOU FOUND IT!!!
Turn off the SPECTROMAX instrument using the on/off switch on the back of the computer.
DO NOT turn off the computer but do close all the open windows.
PRECAUTION: If you haven’t saved your data and another group reads their plate, your data will be overwritten and lost.
DATA COLLECTION
We have provided you with a template called Site_Biolog-calc-template2011sp.xls workbook to organize the recording and analyzing of the carbon source utilization data. You will find this EXCEL spreadsheet template in Resources in your lab SAKAI site in a DATA folder. The folder location details will be specified by your instructor. You will copy and paste your data after each reading into this template, Site_Biolog-calc-template2011sp.xls workbook, by downloading that file to the Desktop of a networked computer. You will have to carefully copy and paste the day's absorbance readings to the template. It is crucially important that you align the right carbon source to its value (don't forget water) and get the right well numbers transferred to the appropriate position on the Worksheet DAY (1, 2,etc) in the template workbook. Note that the Template contains a separate worksheet for each day within the full workbook. Make sure that you fill out the appropriate day (DAY 1, 2, etc). When you are sure that your data has been copied correctly into the appropriate template day, Save AS : rename the template file with your soil sample code and repost the latest template datafile to your data (sub)folder in Sakai (so that your teammates will be able to access the latest data). In addition to submitting the whole template workbook to the appropriate subfolder in the DATA folder in Sakai, you should upload a copy to your personal dropbox in Sakai.
This template is pre-formatted for the calculations you will do from these data. It includes the formulas to average replicate measurements each day and it will automatically subtract the background (readings in the water wells). There is a normalization for background that will be subtracted automatically (this threshold absorbance is 0.25 for each carbon source). We hope that including these pre-made calculations in the template will make your data analyses for AMR and CMD relatively simple to accomplish. Please make sure you understand these calculations.
Calculating Average Metabolic Response (AMR) and Community Metabolic diversity (CMD)
CALCULATING AMR: Average Metabolic Response over time and between soil sites and Average Carbon Source Utilization Ccore by the microbial community
Average Metabolic Response(AMR)= Σ(mean A590nm of triplicate carbon source tests - Mean A590nm of the three control wells) and less a threshold absorbance of 0.25nm / 31 (the number of carbon sources tested)
The calculation of AMR provides a method to compare total metabolic activity by a soil community among aerobic or facultative microbes. The AMR is calculated as the average difference between A590nm values of the three replicates of each C source and the 3 water control wells. The following calculations were built into the Excel template workbook: Take the average of each of the 3 replicate wells and subtract the average of the control wells from each of the carbon sources. A background absorbance threshold absorbance of 0.25 is substracted also. If you double click on the cells, you can check to see that the formulas and calculations are correct. The final calculation that is already built in is a division by 31 (the number of carbon source wells).
A high score indicates that there were many useable carbon sources for the community, a low score indicates that few of the carbon sources were able to be metabolized well. Calculate AMR for every date read. On the last page of the template workbook, please fill in the AMR table by day and overall average absorbance. Graph these data. Collect this AMR table data for the other 2 soil sample sites that you lab section collected and graph the 3 sites' average AMR over time as decribed in the next section. How do the three soil communities compare?
CALCULATING CMD: community metabolic diversity. CMD is a simple way to represent the total number of substrates able to be effectively metabolized by the microbial community. It's a measure of diversity in use of carbon sources. CMD is calculated by summing the number of positive responses (wells with a positive A595nm value after all the corrections) at each incubation time. Any negative values are considered 0 absorbance.
On your worksheet enter either a 0 or a 1. Zero indicates that there was a negative value or 0 for corrected A590nm. A 1 indicates a postive value of greater than zero. Once you have entered a 1 or a 0 as the "corrected" absorbance for all the cells, the template will calculate the CMD for that day. Complete the final CMD table on the last page of the template workbook and then collect these data for the other soil sampling sites for your lab section. Graph the average CMD over time as described below. How do the three soil communities compare?
GRAPHING THE DATA:
Both AMR and CMD should be calculated for each measurement date.
Once your team is finished collecting the data, make figures for AMR, CMD, and Carbon Utilization patterns.
To graph AMR: You will plot each soil sample's AMR value on the y axis against time on the x to provide a quick comparison of metabolic kinetics of the 3 worksites.
To graph CMD: Plot the calculated soil sample's CMD values on the y axis versus time on the x to get a sense of community functional metabolic richness.
Carbon source utilization pattern:
Neither AMR nor CMD analysis provides information about the pattern of carbon substrates used in each soil community.
To examine the pattern of carbon sources used across communities, you will need to plot the average A590nmabsorbance on the final day of data collection on the y axis and the 31 different carbon sources on the x axis in one figure that compares all 3 work-sites.
Can you think of another way to compare the patterns of substrates?
Since these data on community physiological profiling will be useful in your final paper, be sure the final completed EXCEL workbook, including the tables and graphs, is submitted to the DATA folder for your soil sample site no later than the start of LAB 5. Carefully label any submitted images or figures so that any student could identify the soil sample site, what is graphed, and who were the author(s), in case questions arise.
Continue Isolation of Azotobacter, Hyphomicrobia, Spore Forming, or other interesting Bacteria
Continue to attempt to isolate to pure culture desired groups of bacteria. Directions found in the Protocols section of the wiki at Culture Media: General Purpose, Selective, Enrichment, Differential, & Assessment of Digestive Exo-Enzymes
Directions for Streaking for Isolation onto new solid media is found at Streaking for Isolation
Coordinate with your partner and other classmates to try to isolate colonies that look different from each other. It would be great if everyone in the class found different bacteria; ideally one Hyphomicrobium, one Azotobacterium, and one spore former. That may not happen, but it will make your project more interesting if you and your teammates have a wide variety of bacterial genera and/or species to look at.
Your goal is for each student to end up with 3-4 pure cultures of DIFFERENT genera of bacteria from as many groups as possible that grow well in their isolation medium or on Nutrient agar.
Once you believe you have pure isolates, continue to subculture to fresh plates each week (isolation streak a colony onto a fresh plate), in subsequent labs you will make a bacterial smear and do a Gram stain and start other tests to explore the physical and metabolic characteristics of the isolates.
The following is repeated from earlier labs since each of you may be at different points in the enrichment and isolation process.
Finding Denitrifying Methylotrophs (Hyphomicrobia) Bacteria :
Use Denitrifying Methylotrophs Medium with and without methanol(DMM)
The bacteria in this group have two very distinct morphological forms (dimorphic): a motile, swarmer cell and a stalked, stationary, prosthecate cell. The motile cell is flagellated, typically with a polar or subpolar flagellum. The sessile cell attaches to the surface of some solid material using either an adhesive or a specialized outgrowth at one pole called a prostheca. Reproduction occurs by budding from the prosthecae and results in one motile and one sessile offspring.
Hyphomicrobia are facultative aerobes and chemoorganotrophs in the alpha-Proteobacteria group. They are common in aquatic environments and in nitrate rich environments. In nitrate-rich non-aquatic sites, such as soil, these organisms rely on other bacteria to degrade local organic molecules to a useable form. They use C1 carbons for nutrients and reduce nitrate to N2 for their energy needs.
In pure culture, you should be able to find both morphologic forms in about equal ratio. Genera of this group we might find in soil include: Caulobacter, Asticcacaulis, Hyphomicrobium, Pedomicrobium, Hyphomonas, and Thiodendron (Find more reference information in The Prokayotes, on-line access available as directed in the Resources section of this wiki).
Isolation is based on their inability to grow on nutrient agar while they have the ability to grow anaerobically in the presence of methanol, while reducing nitrate to N2. (We will use screw cap tubes to reduce the oxygen gas exchange in the culture)
Isolation:
Check that you see 'bubbles" in your secondary enrichment tube of DMMM made in lab 2. Each student will isolation streak from this medium today (IF there are nitrogen bubbles in it) onto one DMM (no methanol) plate. These plates will be incubated in a sealed chamber containing an open tube of methanol at 30C. We will need to change and/or refill the methanol each week. Email her if the methanol tube is less than 1/2 full or, if you don't mind replacing the methanol yourself, add some methanol from the stock bottle in the fume hood---be careful not to split it on your hands, wear gloves, and do this transfer in the fume hood.
Allow these plates to grow in the chamber at 30C until next lab. Examine the streaked plates of DMM using the colony counter or a magnifying glass. Look for tiny, glassy (hyaline) colonies, possibly volcano shaped.
Identification:
Keep streaking for isolation until you are sure that you have pure isolates. Note that other organisms can overgrow the Hyphomicrobia or attach to them so that it is initially difficult to separate them from contaminants in the streak plates. Reduce the chance of picking up contaminants that will attach to your desired bacteria by transferring newly arising colonies as soon as you see them: use an inoculating needle or sterile toothpick rather than a loop. DO NOT touch anywhere on the agar but on the colony you want to subculture. Pick up the bacteria from the center of your colony when transferring them to a new plate. After the bacteria are transferred to zone one of your new plate, you can use your loop to streak out the other zones. Finally, when you feel confident you have a pure isolate of a denitrifying methylotroph, perform an isolation streak on nutrient agar. If bacteria form colonies on NA, you did not have a pure colony of Hyphomicrobia, as these bacteria can not grow on nutrient agar. However, you may have a different group of methylotrophic bacteria; therefore, continue trying to isolate and separate the methylotrophic bacteria to pure culture.
Finding Nitrogen Cyclying Bacteria: Azotobacter
Use Azotobacter Medium
Azotobacter, important nitrogen cycling bacteria, are able to aerobically use N2 as their source of Nitrogen without a symbiotic partner. They use mannitol as their sole carbon source. They are generally Gram-negative, large rods, or ovoid cells.
-
Isolation:
Watch for the appearance of isolated, slimy colonies. - Continue to isolation streak until you think you have pure isolates.
To test for purity from contaminants:
Look for different kinds of colonies on nutrient agar. Make a subculture of your isolate on NA from an isolated colony found on Azotobacter medium. Streak for isolation using your best technique. If there is more than one type of colony (judge by color, shape, and other visible factors)appears on your plate, Gram stain each different looking colony keeping track of which stain came from which colony. Note that Azotobacter bacteria may not appear slimy on nutrient agar so it is hard to recognize which is the colony type you seek. Once you have determined which colony on the NA plate is most likely to be Azotobacteria (from bacterial morphology, arrangement, size, and other characteristics you found when you researched Azotobacteria in Bergey's Manual or The Prokaryotes), then restreak the appropriate colony onto Azotobacter medium and continue the isolation from that plate (discard the NA).
Finding Spore Forming Bacteria
(Such as members of genera Bacillus and of Streptomyces in the Actinomycetes family
using Glycerol Yeast Extract agar for enrichment)
- Perform another check on your plate for colonies, especially if you have not found a colony yet.
- When well-isolated candidate colonies appear, use the tip of a sterile toothpick to pick up a small but visible amount of growth, being careful not to touch anything but the tip of the colony.
- Isolation streak any interesting colonies (preferentially chose those that appear like "little volcanos" or "powdered sugar") onto new glycerol yeast plates (one colony/plate) using your flame sterilized inoculating loop after you have applied the growth from the colony using a toothpick or your loop to Section one of your streak plate.
Spore forming bacteria are highly resistant to environmental stresses and to disinfection procedures. Among these hardy bacteria are members of the genus Bacillus and the Actinomycetes group of bacteria. All of these bacteria, particularly the Actinomycetes genus Streptomyces, are important sources of antibiotics. Actinomycetes often show a uniquely recognizable filamentous and/or leathery colonial morphology [1] that will help you find them.
The genus Bacillus, a member of the Firmicutes is one genus in a large group of Gram positive organisms. Bacillus spp. are known for the ability of the vegetative cell to produce a metabolically inactive state (a spore). It is likely you will find several subgroups of Bacillus growing on general purpose media or other of the media we use. Use web images to become familiar with the colony morphology of Bacillus so that you will recognize likely candidates and choose them for isolation.
We will use oven dried soil on selective media to enrich and select for spore forming bacteria that can survive oven drying and the high osmotic pressure in our selective medium. You should try to isolate and characterize several different appearing colonies from this medium. Do some research on the web to find images of macroscopic colonies and microscopic bacteria from these groups so you will recognize them if you find them.
Glycerol Yeast Extract Agar (GYEA): a selective medium to enrich for many of the spore forming, antibiotic producing bacteria in the Actinomycetes, Bacillus, and other groups of Gram positive spore formers.
Identification:
Actinomycetes colonies may be slow growing, so check your plates every few days for up to 2 weeks. Bacillus spp. are often able to spread across the surface of the agar, so isolation is sometimes difficult. Shorten the incubation time so you can find the colonies when they are still small.
Assignment
Your instructor has made stained slides of the soil extract that you make in Lab 3. You will view them and calculate the total number of microorganisms in your soil sample in this culture-independent assessment of abundance of your soil microbial population. You will compare that number to the culture-dependent assessment of CFUs/gram of wet soil that you obtained from standard plate count made from your original soil extract set up in LAB1. Submit your full calculations) quantitative assessments to your dropbox in Sakai before your next lab meets. The timely submission of the daily BIOLOG-workbook entries to the DATA folder for your worksite is also expected. The calculations for the exoenzyme section of today's lab can be performed in lab or after lab next week.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab11
Lab 12


