BISC209/S11: Lab5
LAB 5: Soil Microbial Diversity &Function
Part 1: Culture-Independent Identification of Soil Bacteria
Your instructor will return your frozen, cleaned-up pcr products containing amplified fragments of 16s rRNA gene from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rRNA gene fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of Escherichia coli bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin.
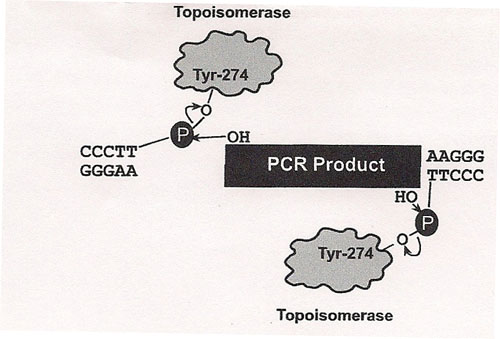
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends.
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit will work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form of E. coli that we will use for separating the amplified 16s rRNA genes from our soil flora.
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death)gene encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. As added insurance that we will select only colonies that are transformed with a plasmid vector with a 16s rRNA gene insert, there is a lacZ gene positioned next to the ccdB gene in the vector. LacZ encodes beta-galactosidase, an enzyme that catalyzes the breakdown of colorless substrates such as Xgal (5-Bromo-4-chloro-3-indolyl beta-Dgalactopyranoside) to a colored cleavage product (in this case, a blue product). Colonies that are transformed with "empty" vectors will not be selected out by plating the colonies on media with kanamycin since the kanamycin resistance gene will be expressed from the empty plasmid vector. However, the promoter for transcription of the ccdB gene AND the lacZ gene is disrupted by the insertion of the 16s DNA insert. Because of this disruption of transcription regulation, the lacZ gene product (beta-galactosidase) and the ccdB product (gyrase poison)are not produced in appreciable quantity. This means that cells containing a plasmid vector with our 16s RNA gene have this disruption of LacZ and ccdB gene regulation and will not be killed by absence of DNA gyrase. They will live and form not-blue colonies because the Xgal in the medium will not be converted to a blue product due to lack of the catalzying enzyme, beta-galactosidase. You will look for white or "not-blue" colonies. (Cool technology!)
Part A: Using Zero Blunt TOPO PCR Cloning Kit with One Shot TOP 10 Chemically Competent E. coli
PCR cloning requires three steps.
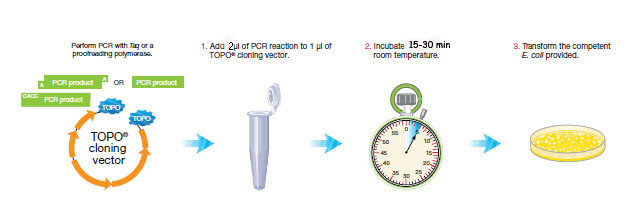
We will clone three pcr products/per sampling site, if your team had three successful amplifications. If you had 4 successful amplifications from your sampling site, use the most successful 16s rRNA gene amplifications and omit the weakest one.
Procedure: Add the reagents in this order!
1. Add 2 μl of PCR product to a 0.2ml pcr tube (your team color)
2. Add 1 μL of salt solution (final conc. 200mM NaCl, 10mM MgCl2).
3. Add 2 μL of purified HPLC water (DNAase free).
4. Add 1 μL of pCR®II-Blunt-TOPO® cloning vector plasmid. (MAKE sure you pipet this correctly with a P2 and a filter tip!)
4. Incubate 15 min at room temperature.
5. Continue to next step: Transform Oneshot Top10 competent E. coli.
Part B Transforming TOPO Competent E. coli
Genotype of OneShot TOP10 Competent Cells: F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG
General Handling: Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting(no vortexing).
Transforming One Shot® Competent Cells
Introduction: Once you have performed the TOPO® Cloning reaction, you will transform your pCR®-Blunt II-TOPO® construct into TOPO10 competent E. coli provided with your kit.
You will need to gather:
In addition to general microbiological supplies (e.g. petri dish with ethanol, glass spreader or sterile glass beads), you will need the following reagents and equipment.
• TOPO® Cloning reaction from Performing the TOPO® Cloning Reaction
• S.O.C. medium at room temp.(included with the kit)
• 42°C water bath
• warm Luria-Bertoni (LB) plates containing 50 μg/ml kanamycin and 50μL/ml Xgal (5-Bromo-4-chloro-3-indolyl beta-Dgalactopyranoside)
• 37°C shaking and non-shaking incubators
Preparing for Transformation
For each transformation, you will need one vial of competent cells and two
selective plates.
• Equilibrate a water bath to 42°C
• Bring the vial of S.O.C. medium to room temperature.
• Warm LB plates containing 50 μg/ml kanamycin and Xgal at 37°C
for 30 minutes.
• Thaw on ice 1 vial of One Shot® cells for each transformation.Don't remove them from the -80C until ready for use.
Transformation Procedure
1. Add 2 μl of the TOPO® Cloning reaction when it is completed into a vial of One Shot® Chemically Competent E. coli and mix gently by swirling. Do not mix by pipetting up and down!
2. Incubate on ice for 10 minutes.
Note: Longer incubations on ice do not seem to have any affect on transformation
efficiency. The length of the incubation is at the user’s discretion.
3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).
4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .
5. Add 250 μl of room temperature S.O.C. medium (it must NOT be cold).
6. Cap the tube tightly and put the capped tube in a empty non-sterile 15 ml. conical tube and shake the tube horizontally (200 rpm) at 37°C for
1 hour. While the shaking is going on, slightly dehydrate 2 LB + kan + Xgal plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then place the plates in the 37C incubator to prewarm. (The plates must NOT be cold when transformed cells are plated.)
7. After the 1 hour incubation of the transformation mix, Use your P200 micropipet to pipet 50 μl from each transformation to the center of a prewarmed LB + kan+ Xgal plate. Using a disposable sterile plastic spreader, carefully spread the aliquot of cells over the entire surface of the plate.
8. Repeat step 7 on a new LB + kan + Xgal plate, using a 200 μL volume of transformed cells. You will plate two different volumes to ensure that at least one plate will have well-spaced colonies.
9. Incubate all plates upside down overnight at 37°C. Remember to label each plate with all the appropriate information: your initials, lab section, date, your soil sample id and habitat, the type of medium, and the id of the cells and volume used. Refrigerate the remainder of your transformed cells at 4C overnight in case you need to plate a smaller number of cells to achieve isolated colonies. Check your transformations after 12-18 hours (overnight incubation)to be sure of successful transformation. When medium size, ISOLATED colonies, have appeared, refrigerate your transformation plates until LAB 5. DO NOT LEAVE THEM INCUBATING TOO LONG, resulting in overgrown colonies that are not isolated! If you have no transformation or a lawn of growth after the initial overnight incubation, contact your instructor immediately for help. You will need to reisolate by plating a diluted or smaller volume of cells on a new plate or redo the cloning and transformation if neither of the transformations from your habitat is successful.
10. An efficient TOPO® Cloning reaction will produce several hundred
colonies. The colonies with inserts will be white or, at least, "not-blue". Look at the map of the cloning vector and the background information description of the cloning and figure out why all colonies should have soil genomic 16s rRNA inserts and why those that are not blue are particularly likely to be the ones we want.
Transformation Media Recipes
S.O.C. Medium
(may be stored at +4°C or
room temperature)
2% Tryptone
0.5% Yeast Extract
10 mM NaCl
2.5 mM KCl
10 mM MgCl2
10 mM MgSO4
20 mM glucose
Luria-Bertoni Agar
1% tryptone<BR.
0.5% yeast extract
1% NaCl <BR.
2% agar for solid medium, none for broth
50mg/L Kannamycin
50mg/L Xgal (optional)
Part II: Culture-Dependent Isolation & Characterization of Soil Bacteria: Obtaining Pure Cultures of Isolates
By this week we hope that you have pure cultures from your enrichment and isolation protocols. If you have isolated, pure colonies on your isolation streak plates, congratulations!. Make sure that these potentially pure cultures have colonies that look like the original source colony and that all of the colonies look the same (they can be of different size). If you are having trouble obtaining well isolated colonies, consult with your instructor.
If necessary continue to attempt to isolate to pure culture desired groups of bacteria. Directions found in the Protocols section of the wiki at Cuture Media: General Purpose, Selective, Enrichment, Differential, & Assessment of Digestive Exo-Enzymes
Directions for Streaking for Isolation onto new solid media is found at Streaking for Isolation.
Your goal is for each student to end up with 3 pure cultures of DIFFERENT genera of bacteria from as many groups as possible.
Once you believe you have pure isolates, continue to subculture to fresh plates each week (isolation streak a colony onto a fresh plate), in subsequent labs you will make a bacterial smear and do a Gram stain and start other tests to explore the physical and metabolic characteristics of this isolate. Generally the medium used is the isolation medium, however, at some point you may want to test the ability of your isolates to grow on nutrient agar. Remember, if you successfully isolated hyphomicrobia your colony should not grow when streaked on nutrient agar. The other cultures may grow as well or better since the nutrient agar we use is rich in nutrients. If your organism grows well on nutrient agar, you can streak on this medium each week and stop using the original isolation medium. Ask you instructor if you are not sure what to do.
If you have pure cultures now, you will continue with the Activities that begin next week --if not, wait until you do have well isolated colonies in a pure culture. If you are unsure, consult with your instructor.
Preparation for Next Lab
Some testing for metabolic and physicial characteristics of your isolates will start next week. You will use the pure cultures that you have started this week or their descendants. Depending on the test, you may need a fresh liquid broth culture, or an isolation streak plate culture. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. The number of hours it takes from inoculation until a bacterial culture moves from log to stationary or death phase depends on its generation time, the conc. of the inoculum, and other factors. If you have a reasonably fast growing culture, you should make a subculture into Nutrient broth medium about 24-48 hours before you inoculate the test medium. Keep track of how fast each of your soil bacteria grow, on which media,and at what temperature. There is no point in trying to make Nutrient broth subcultures of isolates that won't grow in Nutrient Broth. Since this is an investigative lab with no pre-designed outcome, success will require planning and organization as well as copious notetaking.
Reference Information
Your most important resource for looking up information about your isolates will be the reference manuals: THE PROKARYOTES and Bergey's Manual. Wellesley College has these valuable reference books available in electronic form. Link to the electronic edition of | The Prokaryotesthrough Springer ebooks.
Link to the electronic edition of | Bergey's Manualsthrough Springer ebooks.
Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests your perform. It is unlikely we will be able to provide ALL the tests you might wish to perform to evaluate the various roles your soil bacteria may play in its ecosystem, but we will obtain interesting information. The DNA sequencing analysis of the 16s rRNA genes should provide the identity of many of the bacteria in your community, often to the genus and species level.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
M&M: Compose a draft of your Materials and Methods section of your final paper with the following three general sections:
1)Community level physiological testing: carbon source profiling, nitrogen cycling profiling, exoenzymes profiling;
2) Identification of bacteria by 16S rRNA gene sequencing from soil genomic DNA;
3) Selection and isolation of soil community bacteria to pure culture. More information can be found at Lab 5 Assignment: Materials & Methods
Continue following the appropriate protocols to isolate and characterize a few of the culturable bacteria that make up your soil community. In Lab 6-8, you will doing most of the assessment of your isolates' physical and metabolic characteristics through a battery of tests and special stains, a few of which require some preparatory work. Familiarize yourself with the tests and stains you will preform. Make sure you have outlined the protocols in your lab notebook and started any necessary cultures on appropriate medium so that they will be ready to use in lab at the appropriate time:
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab11
Lab 12