BISC209/S11: Lab7
LAB 7: Examples of Co-operation and Competition in a Soil Community: Bacterial Interactions, Quorum Sensing, Functional roles in the Nitrogen Cycle
Status of Culture Independent ID of Bacteria from a Soil Community by 16s rRNA Gene Sequencing
We have sent out the glycerol stocks of the clones that you prepared for DNA sequencing of the 16s rRNA gene. We must also send an appropriate primer. What's the sequence of such a primer? What's going on in those automatic sequencing machines in Danvers? Make sure you understand how chain termination (Sanger) sequencing works. There will be some theoretical questions about this type of information in the lab practical. We should have the sequencing data back by LAB 9 when we are scheduled to meet in a computer lab and learn to analyze the data generated so we can find out, we hope, a lot about the diversity and phylogenetic relationships among the soil bacteria in our soil communities.
Confirmation of Gram stain results by Selective/Differential Media:
Did each of your isolates grow on PEA or EMB? What does that result mean about the isolate's cell wall composition? Did you confirm your Gram stain findings?
Testing for Examples of Co-operation and Competition Among your Cultured Isolates
Antagonistic and Mutualistic Interactions
- NOTE: You must remember to set up fresh nutrient broth cultures for your isolates 1-3 days before lab to do this test!
The microbial community living in soil is a complex one with many different microorganisms. As is true of any environment, these microbes interact with each other - both functionally and physically. Today, you will be using your cultured isolates to test for possible examples of mutualism or antagonism (co-operation or competition). Do selected bacteria from your community help each other or harm each other while trying to find a niche in your soil community? You will culture them in controlled communities to attempt to detect positive or negative interactions. Some of these bacteria may prevent the growth of others through the production of chemical inhibitors; others might promote the growth of their neighbors by producing metabolites that are needed.
Interaction Assay
1. You will only be using 64 of the wells on a 96 well plate for this exercise. Each team member will select two or three of her isolates to combine with others in the soil community. There will be a total of 8 isolates tested for interactions per soil sampling site. The isolates chosen must grow on nutrient agar (general purpose medium). Use the Excel template provided Media:template.xls to record the selected isolates identifying code on the well(s) where they will be inoculated. You will inoculate wells A1-A8 and B1-H1.
2. Using a sterile pipet tip and your P200, inoculate 50 µl of the isolate grown in fresh nutrient broth culture into your assigned well(s).
Note that the isolate inoculated into well A1 will not be inoculated into any other wells. All other isolates will be inoculated into 2 different wells. For example if you inoculate isolateX into well A2, that isolate will also be inoculated into well B1. Likewise, the isolate placed into well A3, will also be placed in C1 etc. FOLLOW THE TEMPLATE CAREFULLY!!!!!! It is easy to get this inoculation messed up, but don't!
3. Use the P200 to add another 100 µl of sterile nutrient broth to the wells that were inoculated in step 2 in order to dilute the cultures.



4. When you are finished loading the top and side wells, use the P20 micropipette to move 10 µl from the top wells A2 - A8 into wells B2 - B8 moving top to bottom. Continue loading A2-A8 to C2-C8, etc. DO NOT TRANSFER ANYTHING TO THE WELLS A1, B1, etc.) You do not NEED to change the tip as you fill each empty well in a column (e.g. B2-H2) unless you think you might have contaminated your tip. Repeat this transfer until all the rows have 10 µl of the inoculum from A2-A8. (Note: if an eight tip multichannel 10 μL pipet is available, you could use it to fill the empty wells as long as you remove the tip on the first channel before pipetting).

4. Now we will mix the isolates in wells A1 - H1 with isolates in the other wells moving left to right.

5. Using the P20, take 10 µl from wells A1 and mix into the wells A2 - A8 moving left to right, changing the tip on the pipet each time (in case it touched the existing solution in the well). NOTE: if a multichannel pipet is available, you could use it to inoculate the wells (using all 8 channels).
6. Repeat this process until you reach A8-H8 and have 20 μL in each well (except the top and side wells.
7. Each of your wells should now have isolates growing by themselves (A1-H1 and the diagonal wells), as well as isolates mixed together in all the other wells.
8. We will inoculate from this plate onto a square tray containing nutrient agar medium. For this step we will use either a tool called a "frogger" or a multichannel micropipette. If using the frogger, dip the tips into 96 wells to attract a drop of inoculum onto the end of each steel tip and then touch the those tips to the surface of the sterile NA square NUNC plate. Do not break the surface of the agar but make sure your pressure is even so every steel tip has touched the agar surface and deposited the same inoculum. Be sure to disinfect the frogger by dipping it into a series of disinfectant and rinse solution.
Instead of using the frogger, if a multichannel pipet is available, set it to 5µl and remove 5μL of culture from each well of your culture dish and deposit all of it onto an area of the NA square agar NUNC plate that is in the same location as in the 96 well culture dish. Repeat this until you have completed depositing the full array and that it mimics the look of your culture dish.
7. Wait for your inoculated spots to dry before placing your tray upside down at Room Temp to incubate for a week. You should come in to the lab a few times this week to check on your assay and note any differences in the appearance of the colony growth of each isolate, alone vs mixed. Note that the inoculum in the diagonal spots is actually a single isolate. Note the "edge" effect, a difference in the appearance of the colony growth in the spots along the perimeter of the plate as opposed to those growing in a more protected locations (the diagonal control colonies).
Bacterial Quorum Sensing
Quorum sensing - chemical signaling within our community
Many bacteria are able to secrete signals into their environment to sense their density. Since bacteria are single-celled organisms, why would it be important for them to sense density? A very well studied example of a quorum sensing system was discovered in Vibrio fisheri, a bacterium that produces light only at high densities. Because the light produced by a single bacterium is unlikely to be detectable, it makes sense to wait until a "quorum" is reached before turning on the expensive metabolic pathway that creates light. In this way, a gene regulatory network is actually controlled by cell density. To hear more about it from another source, visit this YouTube video: Bonnie Bassler.
Today we will be setting up a test to see if any of your isolates are secreting Auto-Inducer (AI) into the surrounding media. Specifically, we will be using a strain of bacterium called Chromobacterium violaceum. This organism is a Gram-negative coccobacillus and a facultative anaerobe that is normally found in the soil. It produces a very strong purple pigment (hence the name) in response to AI. (This pigment called violacein, may be useful for the treatment of colon and other cancers.) C. violaceum grows readily on nutrient agar, producing distinctive smooth low convex colonies with a dark violet metallic sheen. Its full genome was published in 2003.
We will also use a violacein-negative, mini-Tn5 mutant of C. violaceum (CV026). This mutant can produce pigment in response to the AI from other bacteria, but can no longer secrete its own AI - this will be our biosensor.
1. You should have prepared for this assay by making a recent subculture streak plate of each of your isolates on Nutrient agar (IF THEY WILL GROW ON NA!). These fresh sub-cultures should be no more than 1 week old when starting this test (unless an isolate that you want to test is a very slow grower). Throughout this procedure be sure to use good aseptic transfer technique. The stress of a hot loop can cause the transposon in the mutant strain to be lost from the cells so we will use either plastic sterile loops or sterile toothpics for the Chromobacterium colonies. When transfering your isolates, remember to flame and cool your loop BEFORE YOU touch any colonies.
2. Use a marker to draw a line on the bottom of the plate dividing the plate into two halves.

3. Label one side of the center line on a plate of nutrient agar with the name of the positive control bacterium, Chromobacterium violaceum ATCC 12472 (the parent strain to CV026) and label CV026 on the other side of the line.
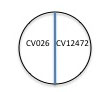
4. On all the other plates (one for each isolate to be tested), label CV026 on one side and your isolate code name on the other side of the line.

5. Begin with the young culture of CV026. Use one sterile plastic loop to inoculate this organism onto the labeled CVO26 side of all your plates. As long as you work carefully, use the same loop to pick up a visible amount of growth on the source plate to deposit inoculum on all the plates. If you think you may have contaminated the loop, obtain a new loop and continue. Streak the inoculum near but not touching the center line that you drew on the bottom of your plate. Repeat this procedure until all the plates (one for each isolate to be tested) have been inoculated with CV026.

6. Use another sterile plastic loop to inoculate the control plate that you labeled in step 3. Streak the inoculum of a fresh culture of ATCC 12472 on the side of the plate that doesn't have CV026.

7. Use your wire loop, making sure it cools after flaming, to streak each of your isolates on the other side of the labeled plates, near but not touching the center line.

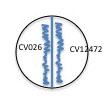
8. Place your plates upside down and incubate them at RT for a week. If the isolate produces AI and it is sensed by CV026, a purple pigment will be produced by the rescued mutant.
The Nitrogen Cycle: Do you have examples of isolated bacteria from your soil community that co-operate to complete the nitrogen cycle in a soil community?

Many soil bacteria can fix nitrogen by reducing N2 to any or all of the other forms of nitrogen in the cycle: nitrate, nitrite, and ammonia. Other soil bacteria can break down organic nitrogenous molecules to ammonia, nitrite, or nitrate to N2, allowing the cycle to run in both directions simultaneously. As a community, soil bacteria work co-operatively to provide crucial metabolic raw materials for each other and for plants, animals, and other organisms that share their habitat.
To provide evidence for this type of co-operative mutualistic behavior in your soil community, we will test the isolates that appear to be denitrifiers (Hyphomicrobia) or nitrogen cycling bacteria (Azotobacter) to see if they can produce some of the by-products of nitrogen reduction or break down nitrogenous molecules. What's your hypothesize about the role the Hyphomicrobia and the Azotobacters have in the nitrogen cycle aerobically.
1. Use a sterile loop to aseptically transfer a visible amount of growth from a fresh well-isolated colony of putative nitrogen cycle contributors growing on PYC or NA medium to a small glass sterile tube with 2ml of sterile water. Mix well.
2. Compare the turbidity of this inoculated tube to a #5 McFarland turbidity standard available at the instructor’s bench. Add more water or more culture until the turbidity appears to match the standard.
3. For each isolate to be tested, label 3 screw capped and 3 loosely covered 16X100mm tubes that are 3/4 full of peptone meat extract medium (PM - Recipe: 0.5%(5 g/L) peptone; 0.3% (3g/L) meat extract; pH 7.)
4. Aseptically transfer 200μl of the culture made to appropriate turbidity to each of 3 sterile screw cap tubes and to the 3 loosely capped tubes.
5. Tighten the caps on the screw cap tubes leaving minimal air-liquid interface.
6. Test one of the loosely capped tubes immediately for the presence of ammonia, and nitrate/nitrite using test strips. Directions for using the test strips are detailed below.
7. Incubate all the cultures at 30°C.
8. Prepare a data sheet for your team’s tested isolates. Arrange a schedule with your teammates for someone to come in to test all the cultures each day for ammonia, nitrate, and nitrite. Record the results each day on the team data sheet and post it to your team's DATA folder in Resources in Sakai so your teammates can access it. Include a column or row on your data sheet to record observations (such as when you detect evidence of growth by seeing an increase in turbidity compared to the McFarland standard, any change in smell, color, or appearance of the cultures).
PROTOCOL for TESTING: Ammonia, and Nitrite/Nitrate
The detection limit of these strips is: Ammonia (6ppm - mg/L)), nitrite (10ppp-mg/L), and nitrate (200ppm-mg/L).
1. Avoid contaminating your cultures by aseptically removing about 500ul of culture solution and placing it in a small empty non-sterile test-tube. Discard any used tips in an orange biohazard bag.
NOTE: You can use the same 500μL aliquot to dip all the strips in each day but you will need to remove a new aliquot each day that you perform the test. Do NOT replace the tested aliquot back into your original culture. Be sure you have organized a good system for recording the data daily.
2. Carefully remove one ammonia test strip at a time and reseal the container.
3. Dip the ammonia test strip in the culture aliquot first. Time for 10 seconds, then remove the strip slowly so it will not drip.
5. Use the color chart on the bottle to compare the color of the strip and determine ppm (mg/L).
6. Discard the strip in an orange biohazard bag immediately.
7. Carefully remove a nitrate/nitrite test strip from the container and reseal the container.
8. Dip the strip twice into the same culture aliquot. Remove the strip so the pads face up.
9. Do not shake the strip.
10. Wait 60 seconds.
11. Determine the concentration (ppm = mg/L) of both nitrate and nitrite by comparing the color of the strip using the color chart on the container.
12. Discard the strip in the biohazard bag.
13. Place the small tube with your 500 μL aliquot in a rack in the clean up area next to the sink to be autoclaved.
14. Replace the cap tightly on the screw capped tube and return both tight and loose capped cultures to the 30C room.
15. Go to the instructor's computer and find your team's data sheet in the DATA folder in Resources in Sakai. Add today's data including observations and upload the file to your data folder.
Possible examples of reacting to competition: Continue Antibiotic Production test started last week
Week 2
Need fresh control cultures of Eschericia coli (Gram negative), Staphylococcus epidermidis (Gram positive) and Micrococcus luteus (Gram positive) grown in nutrient broth to the same turbidity standard used last week for isolate cultures.
PROTOCOL
Use the cultures of your isolates set up last week on NA.
Use a sterile swab to aseptically apply parallel lines of inoculation of each of the control broth cultures of : E. coli, Micrococcus, and S. epidermidis as shown in the illustration below. Use a different sterile swab for each culture. These parallel inoculation lines should be made perpendicular to the putative antibiotic producer's (your isolate's) colony growth. (See the illustration.) Be careful not to touch the putative antibiotic producer's growth with the control cultures, but come as close. Make a template in your lab notebook and label the plate to indicate where each control culture is streaked. Incubate these culture plates at RT for a week. One person/lab should also set up viability controls by swabbing each the three broth cultures onto separate areas of another NA plate. If you see growth of each of the controls next week, we will be sure that any inhibition of growth is due to sensitivity to a diffused antibiotic rather than lack of growth occurring because one or more of the control broth cultures you used today lacked enough viable cells to form colonies on your test plate.

Other Physical & Functional Capabilities: SIM test
Every isolate should be inoculated into a SIM tube. This test gives information about motility and about two other metabolic capabilities: hydrogen sulfide production and the presence of the enzyme tryptophanase.
A full description of these tests can be found in the protocols section: Motility Tests.
If your SIM motility test is positive when we analyze the results in Lab 8, you will be able to confirm motility by performing a hanging drop motility test and, if that is positive, trying the flagella stain.
PROTOCOL:
Inoculating the SIM tubes involves a technique you have not yet practiced. You will use an inoculating needle: the wire extending from the handle of the needle will not have a loop on the end.
1) Flame sterilize an inoculating needle, cool it for a few seconds, and pick up a visible amount of your isolate on the tip.
2) Stab the needle with the inoculum deeply into the center of the medium in a SIM tube, stopping just before the bottom of the tube or, if you are running out of needle, stab it until the you are almost to the end of the needle.
3) Withdraw the needle through the same inoculation channel. (This procedure is also known as "making a soft agar deep".)
4) Inoculate E. coli as a control into another SIM tube using the same technique.
5) Incubate both tubes for 24-72 hours at room temp.
6) After 72 hours of RT incubation, refrigerate both cultures until Lab 8. We will analyze and develop the tests then.
Control Organisms:
| Organism | ATCC | Motility | H2S | Indole |
|---|---|---|---|---|
| Escherichia coli | 25922 | + | - | + |
| Salmonella choleraesuis subsp. choleraesuis serotype Typhimurium |
13311 | + | + | - |
| Shigella flexneri | 9199 | - | - | - |
Microbiologists of previous generations had to do their bacterial identification exclusively from physical and metabolic tests. The tests we are performing in the course of our investigation are a tiny subset of all the morphologic, metabolic, and other tests that you could perform on your isolates to try to identify them through a battery of tests for different metabolic capabilities and characteristics. Be very glad that you are training as a microbiologist in the era of genomics!
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
Write part of the Results section of your final paper on Bacterial Abundance & Diversity in a Soil Community: Co-operation and competition
Please use the results you have obtained so far to assess the abundance and diversity of microorganisms in a soil community found in an artificial Tropical habitat in the Wellesley College Greenhouses. Include evidence for co-operation and competition among members of this community. Please design and use effective figures/tables with appropriate legends that show a new reader how your data answer some of our experimental questions (remember you don't yet have all of the data so some of these questions can't be answered fully or even partially yet): What microbes compose our soil community? Is the community diverse? How so? How are they related phylogenetically? How many microbes are there? How do they function as a community co-operatively and/or competitively?
There are more instructions for this assignment found at: Assignment: Partial Results section with Fig/Tables. Refer also to the appropriate sections in the extensive handout, "Guidelines to Scientific Writing" found in the Resources section of the wiki. Your instructor is also available for guidance, and there are science writing peer-tutors, hired and supervised by the Writing Program, available for writing help. See the Writing Program web page for hours and availability or to schedule an appointment at | http://www.wellesley.edu/Writing/Program/tutors.html.
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab11
Lab 12
