BISC209/S12: Lab5: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| Line 125: | Line 125: | ||
5. The PCR product is now purified and ready for use in DNA sequencing or other primer extension applications.<BR> | 5. The PCR product is now purified and ready for use in DNA sequencing or other primer extension applications.<BR> | ||
6. Treated PCR products should be stored at -20C until required.<BR> | 6. Treated PCR products should be stored at -20C until required.<BR> | ||
'''NOTE: ExoSAP-IT reagent must be stored in a non-frost free freezer!''' | '''NOTE: ExoSAP-IT reagent must be stored in a non-frost free freezer!'''<BR><BR> | ||
==Part E: Submitting Amplicons for Identification == | ==Part E: Submitting Amplicons for Identification == | ||
Revision as of 05:55, 2 March 2012
LAB 5: Soil Community Bacterial Member Identification by 16s rRNA gene sequencing
By this week we hope that you have pure cultures from your enrichment/selection and isolation work. If you have isolated, pure colonies on your isolation streak plates, congratulations!. Make sure that these potentially pure cultures have colonies that look like the original source colony and that all of the colonies look the same (they can be of different size). If you are having trouble obtaining a pure culture, consult with your instructor BEFORE LAB 5. We can't identify your isolate by 16s rRNA gene sequencing if it is not a pure culture.
Part A: Prepare Lysates from pure cultures of Bacteria of Interest
1. Each student will sequence DNA from 4-7 unique bacteria (up to 24 per team). Use well isolated colonies from your best plate (one that's unquestionably a pure culture) for each of your bacteria of interest. Consult with your partners to make sure that your team is identifying bacteria that are unlikely to be of the same species and NOT likely to be mold or nonbacterial microbes.
2. Each students needs pcr tubes of your team color (0.2ml vol.). You will need one for each of your isolates. Wear new gloves when you take them out of the jar on the instructor bench and label each with a unique code to indicate each isolate you want to id. DO NOT CONTAMINATE THE INSIDE OF THE CAP with your skin flora. Use your sampling habitat id (site A, B, C, or D) and a unique set of numbers that your lab instructor will assign each student. Numbers will be found on the board.
3. Using your P20 micropipet, pipet 20μL of sterile water with 0.05% Non-idet P40 into each of the prelabeled pcr tubes. Nonidet-P40 is a detergent that keeps hydrophobic domains dispersed and, thus, helps to solubilize membranes. It is similar to Triton-x 100.
4. Touch a well isolated colony from a pure culture with a sterile and cooled inoculating needle or a sterile P10 micropipet tip (the tiny ones, not the P20 tips) and resuspend a barely visible amount of bacteria adhering to the tip by swishing the tip around in the appropriately labeled tube with water and detergent. Resist the urge to pick up too much cell material, but be aware that some of your isolates, particularly those dry, powdery Actinomycetes (like Steptomyces) are hard to get any cells to adhere. For those, it is ok to take a bit more. The tinest bit is enough, but make sure there is some part of the colony going into the lysate. Putting in too many cells can inhibit the pcr reaction and be as bad as putting in none.
5. Repeat for your other isolates-- into separate tubes.
6. Boil all samples for 5 minutes. This will lyse the cells and inactivate bacterial enzymes. You can boil in a heat block or in the thermal cycler-- if your instructor setup a program to boil and you used the small pcr tubes. Since she did and you did, we will boil the tubes in the thermal cycler today. Be careful when you remove the tubes from the thermal cycler. Point them away from you and ease the lids open while still covering the outside of the tops with your gloved fingers. Do this slowly and carefully with the opening pointed away from you. You don't want the caps to pop and make an aerosol of your bacteria and you don't want to lose your lysate.
Part B: PCR AMPLIFICATION of 16s rDNA from lysates prepared above
Note: We are using a kit from New England Biolabs called Phusion® High Fidelity PCR Master Mix with HF Buffer | http://www.neb.com/nebecomm/products/productM0531.asp. Because you are using a kit to amplify the bacterial 16s rRNA gene and the manufacturer does not have to disclose how to make each reagent from scratch, you can not include typical instructions for making stock reagents (ingredients and concentrations)in your Materials & Methods section. However, since the manufacturer's website includes instructions for how to use this product, you can refer your reader to that information and explain simply that you used this kit to accomplish the gene amplification according to manufacturer instructions. Keep in mind that you WILL have to give specific information about the gene template, the primers (DNA sequences), and the thermal cycler program since those things are not universal but specific to the DNA we want to copy. (Size and GC content are crucial variables).
All reagents for the pcr should be kept on ice and the master mix should be thawed on ice. Since DNA polymerase can function at room temp, we don't want the reaction to start until all the tubes are in the thermal cycler.
The components below have been aliquoted and prepared for you and are in pcr tubes of your team color. Label a pcr tube for each of your lysates with a fine point Sharpie on the top and side of the tube with the unique identifier for each bacterial isolate. We will set up one tube per lab as a neg control.
Setting up the PCR Mix
WEAR GLOVES AT ALL TIMES AND DON'T TOUCH THE INSIDE OF THE TUBE CAPS OR YOUR PIPET TIPS--Always use a new tip when going into anything in a pcr reaction. (Contamination is a significant problem in pcr)
Using a P2 or P10 and filter tips (remember that the P2 has two red decimal place volume indicators while the P10 only has 1 red decimal place indicator. MAKE SURE YOU HAVE DIALED IN THE CORRECT VOLUME!), add 4 microliters of your boiled lysate (containing the template DNA) to the prealiquoted 46 microliters of master mix (contains DNA polymerase, dNPTs, MgCl2, and buffers), primers and nuclease free water mixture described above (for a total volume of 50 μL) in clearly labeled pcr tubes of your team color. Make sure you label on both the top and sides of the tube. The tubes are tiny (holds only 0.2ml vol) and it is hard to write on them legibly but doing so is very important. You will have to use a unique identification code for your isolates and keep the key to the code in your lab notebook (also give a copy to your instructor).
Component TABLE
| Component | amt. in a 50 μl reaction |
Final Conc. |
|---|---|---|
| Purified DNAase free Water |
16 μl | |
| 2x Phusion Master Mix | 25 μl | 1x |
| 27F primer | 2.5 μl | 0.5 μMolar |
| 1492R primer | 2.5 μl | 0.5 μMolar |
| template DNA | 4 μl | 50-250ng optimum is 100ng of DNA/reaction |
Hold the tubes on ice until your instructor tells you the thermal cycler is ready to be loaded. Wipe the outside of the tubes to remove all ice and water before placing them in the thermal cycler.
For the negative control one person will add 4 microliters of water (in place of the template DNA). When you have mixed your DNA or water into the pcr mix by tapping VERY LIGHTLY or flicking to be sure that all reagents are mixed and not adhering to the tube wall, take your tubes to the thermal cycler when your instructor says it's ready. Keep them on ice until then, but wipe off the bottom of the tubes before putting them into the machine. Make a template key in your lab notebook as to where in the thermal cycler you put your tubes.
The thermal cycler program is, generally, similar for all pcr reactions, but the annealing temperature (melting) is dependent on the primer pair. When you design primers, the primer melting temp. can be calculated based on the GC content and other factors. Think about which would be harder to denature: GC pairs or AT pairs and why? For 27F and 1492R, a range of 45-55C is ok, although higher temp. may lead to increased specificity that excludes some organisms' DNA from being amplified.
The length of the fragment you are amplifying determines the extension time. A general rule of thumb is to use an extension time of 1kb per minute. Here, we amplify with primers designed for the 27th and 1492th positions in the 16s rDNA gene region. Therefore our fragment is expected to be about 1.5kb long, so we will use an extension time of 1.5 minutes per cycle.
Thermal Cycler Program:
3 step program
| Cycle Step | Temperature | Time | # of Cycles |
|---|---|---|---|
| Initial Denaturation | 98C | 30 sec. | 1 |
| Denaturation Annealing Extension |
98C 55C 72C |
10 sec 30 sec 30 sec |
35 |
| Final Extension | 72C 4C |
10 min Hold |
1 |
The pcr will run for 45min or so.
Part C: Agarose Gel Electrophoresis of Clean PCR PRODUCT
To see if you successfully amplified the 16s rRNA gene and not anything else, you will "run a gel" on your cleaned pcr products. To run a gel means that we will perform an electrophoretic separation of the DNA fragments in your cleaned up pcr product, using 1/10 vol. of your pcr product applied to a 1% agarose gel stained with Sybr Safe DNA stain. Your instructor will photograph the gel, label it with your amplicon id from the template and post the gel photo to the Data folder in Resources in Sakai so you can evaluate your success at 16S rRNA gene amplification. You should see a single band of ~1.5kb indicating that the only dsDNA in your pcr product came from amplification of a ~1500bp gene fragment. Can you explain how we know the size of our amplified gene fragment?
Your agarose gel is made of 1.0% agarose solution (w/v) in 1x TGE buffer (10x=0.25 Tris, 1.9M Glycine, 13mM EDTA) with SybrSafe™ stain.
DNA is uniformly negatively charged and will,therefore, move toward the positive electrode. The separation is determined by the size or mass of the molecule or fragments of DNA.

Procedure for Agarose Gel Electrophoresis of PCR products
Load 1/10 of the total volume of pcr product (1 microliter minimum). In our case we should load 5 microliters.
You will put the 5 microliters of your pcr product as a spot on a small piece of parafilm and add 5 microliters of well mixed loading dye (0.25% XC, 30% glycerol, 0.1mg/ml RNAase). Mix by pipetting up and down before loading all 10 microliters into a lane of the 1% agarose gel (1% wt/vol in 1xTGE buffer with Sybr Safe DNA stain. Sybr Safe is a proprietary reagent from Invitrogen that cross-links a fluorescent compound to DNA and allows DNA to be visualized under UV light. It is prepared and used according to manufacturer's directions http://www.invitrogen.com). Apply your samples to the wells that you have signed up for on the gel template. Be sure to leave the first two lanes and the last lane empty for the 100bp ladder, the positive control and the negative water control.
Loading dye contains glycerol to keep our sample in the lane rather than floating away and will have one of 3 marker dyes (bromophenol blue, xylene cyanol, or
orange G) that facilitate estimation of DNA migration distance and optimization
of agarose gel run time. 1x TGE buffer is used in this electrophoretic separation (25mM Tris, 0.19M glycine, 1.3mM EDTA. The gel will be run at 120V for approximately 30 minutes.
How will you judge a successful amplification? How many fragments and of what size do you expect to see?
Make sure you give back the rest of your soil DNA isolate and the rest of the cleaned up pcr product to your instructor to freeze after the gel is loaded. Both are now in identical looking microfuge tubes with volume being the only visible difference. Make sure it is clear which is the pcr product and which is the genomic DNA isolate.
Make sure each of your pcr products is clearly labeled with your initials, lab section (Tues or Wed), soil sample identifier code letter, your unique code information for each bacterial isolate and the conc. of the DNA in ng/μL.
Your instructor will photograph and label the gel according to the template you have filled out and post the results to the data folder in Resources in Sakai.
Part D: PCR Purification (Clean-Up)
It is important that your pcr product be free of left over DNA polymerase, primers, and free nucleotides before a sequencing reaction is attempted on it. Your instructor will let you know if you will do this purification today after you have loaded part of your sample on the gel or if she will do it for you later, or, perhaps, we might have the sequencing facility do it for us. The important thing for you to know is that it must be done and that there are several methods commonly used to accomplish this purification. One of the simplest is an enzymatic teatment. If we do this clean=up in house, we will use an Exosap-it® kit from USB.
USB ExoSap-iT PCR Product Cleanup (Product Number 78200)
Protocol
ExoSap-IT reagent treats PCR products ranging in size from less than 100bp to over 20kb with no sample loss. The clean-up reagent is proprietary, but it is based on Exonuclease I and Shrimp Alkaline Phosphatase. Recombinant (rSAP) is added directly to the PCR product to degrade primers and to dephosphorylate dNTPs that were not consumed in the reaction. Add EXSAP_IT reagent directly to the reaction products following PCR. Since ExoSAP-IT reagent is active in commonly used PCR buffers, no buffer exhange is required.
1. Remove ExoSAP-IT reagent from -20C freezer and keep it on ice throughout this procedure.
2. Mix 5μL of a post-PCR product with 2μL of ExoSAP-IT reagent for a combined 7μL reaction volume. NOTE: When treating PCR product volumes greater than 5μL, simply increase the amount of ExoSAP-IT reagent proportionally.
3. Incubate at 37C for 15 minutes to degrade remaining primers and nucleotides.
4. Incubate at 80C for 15 min. to inactivate ExoSAP-IT reagent.
5. The PCR product is now purified and ready for use in DNA sequencing or other primer extension applications.
6. Treated PCR products should be stored at -20C until required.
NOTE: ExoSAP-IT reagent must be stored in a non-frost free freezer!
Part E: Submitting Amplicons for Identification
Before your next lab, your instructors will prepare a 96 well plate of your pcr products that showed successful amplification of the 16s rRNA gene. We will send these samples to a commercial DNA sequencing facility that will "clean-up" our pcr products by removing primers, polymerase and unused dNPTs that might inhibit the reaction. They will optimize the pH and the concentration of the DNA for chain termination sequencing of your amplified 16s rDNA (16s rRNA genes). The sequencing reaction will be done for you and the resulting DNA fragments will be separated by size in an automatic sequencer with a laser that can read the fluorescent tags on the end of each fragment. This crucial part of identification of bacterial members of your soil community will be done for you and the DNA sequences will be reported back as .ab1 files that will require special software to analyze. We want you to understand exactly what's going on in those automatic sequencing machines. Make sure you understand how chain termination (Sanger) sequencing works. We should have the sequencing data back by the time we are scheduled to learn how to analyze the data so we can find out, we hope, the identity of a few members of the bacteria in your soil community.
Cultured Bacterial Isolates: Examples of Competition and Co-operation in a Soil Community
Testing for Antibiotic Production
Start the Testing for Antibiotic Production.
Many microbes secrete antimicrobial compounds to help them compete with other microorganisms for habitat niche. Common antibiotic producers are the Actinomycetes (including Streptomycetes species)and many of the Bacillus species; although these are just a few among many, many antibiotic producing bacteria.
(This testing will take 3 weeks.)
Week 1:
Since the soil is the main source of microbes that supply the world's antibiotics, it's possible that you might discover the next great antimicrobial drug and get very rich by selling the patent for your discovery to a drug company. (Remember that the discovery of penicillin was completely accidental.) In order to find out if any of your isolates are potential antibiotic producers, we will grow your isolates for 1 week on NA before we add the control bacteria or other isolates that may be sensitive to the antibiotic produced and secreted into the medium by the first isolate. You should test all of your isolates for antibiotic production. Be espectially sure to test any isolates that grew originally on glycerol yeast extract medium, because those bacteria are likely to be spore formers. Endospore formers are often antibiotic producers.
1) To begin the antibiotic testing protocol, you will use the fresh broth culture of each of your isolates that you prepared prior to lab.
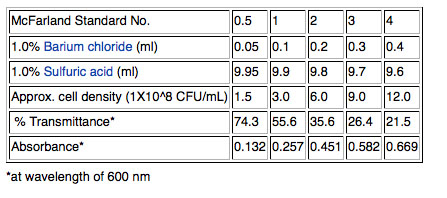
2) Although cell density can be measured more accurately using a spectrophotometer to measure optical density (OD) at 600nm, we will use a quicker method that will work well enough for our purposes. Pipet one ml of the broth culture into a sterile glass tube and adjust the concentration of the cells by matching by eye the "cloudiness" (turbidity) of the cells in the glass tube to a standard provided by your instructor. We will use a McFarland 0.5 standard; the 0.5 refers to the approximate concentration of organisms in solutions which is 1.5X108 cfu/mL for the 0.5 standard. Other common standards are shown in the table below.
3) Add bacteria from a colony of the same isolate grown on solid medium to concentrate the cells in your tube if your culture seems too dilute or add more sterile Nutrient broth to dilute the culture aliquot if it is already cloudier than the standard. The ending volume is irrelevant. Vortex to mix.
4) Using a sterile swab, dip the swab in the concentration adjusted culture tube and make an inoculation (as shown below) down the middle of a plate of nutrient agar.
5) Make a plate for each isolate to be tested. Label the plates carefully and incubate them for 1 week at RT.
Characterization of Soil Bacteria: Maintaining Pure Cultures of Isolates
Continue to subculture your pure isolates to fresh NA plates each week (isolation streak a well isolated colony onto a fresh plate), in the next lab you will make a bacterial smear and do a Gram stain and start other tests to provide examples of co-operative and competitive community behavior among the members of your soil community.
Preparation for Next Lab
Tests of your isolates will continue next week. You will use the sub-cultures made today or their antecedents or descendants. Depending on the test, you may need a fresh liquid broth culture, or an isolation streak plate culture on NA. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. The number of hours it takes from inoculation until a bacterial culture moves from log to stationary or death phase depends on its generation time, the concentration of the inoculum, and other factors. If you have a reasonably fast growing bacterial strain, you should make a subculture into Nutrient broth solid and liquid medium about 24-48 hours before you are to set up a new test. If you have a particularly slow grower, those cultures need to be set up earlier than that. Keep track of how fast each of your soil bacterial isolates grows and plan accordingly.
Reference Information
Your most important resource for looking up information about your isolates will be the reference manuals: THE PROKARYOTES and Bergey's Manual. Wellesley College has these valuable reference books available in electronic form. Link to the electronic edition of | The Prokaryotesthrough Springer ebooks.
Link to the electronic edition of | Bergey's Manualsthrough Springer ebooks.
Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests you will perform or to look up the usual characteristics of the isolates that are identified by 16s rRNA gene sequencing.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
M&M: Compose a draft of your Materials and Methods section of your final paper with the following general sections:
1) Obtaining a soil sample & making a soil extract
2) Enummeration of microbes in a soil community;
a) Cuture-Dependent Colony Count
b) Culture-Independent Direct Count
3) Identification of bacteria in a soil community by 16S rRNA gene sequencing;
a) Isolating Bacteria of Selected Groups to Pure Culture
b) Amplifying the 16srRNA gene by colony pcr & preparation for Gene Sequencing
4) Soil Microbial Co-operation & Competition:
a) Culture-Dependent Microbial Community Assessment of Functional Metabolic Diversity
1) Carbon source utilization pattern and diversity
b) Co-operation and Competition among Cultured Bacterial isolates from a Soil Community
1) Prevalence of Starch, Cellulose, and Phosphate digesters or processors
More information can be found at Lab 5 Assignment: Materials & Methods
In Lab 6-8, you will doing most of the assessment of your isolates' through a battery of tests and special stains, a few of which require some preparatory work. Make sure you set up a fresh broth culture of appropriate media for each isolate a few days before your next lab and have a fresh streak plate of each isolate ready for next week's tests. Familiarize yourself with the tests and stains you will perform. Make sure you have outlined the protocols in your lab notebook and started any necessary cultures on appropriate medium so that they will be ready to use in lab at the appropriate time: