BISC209/S12: Lab5
LAB 5: Soil Community Bacterial Member Identification by 16s rRNA gene sequencing
By this week we hope that you have pure cultures from your enrichment/selection and isolation work. If you have isolated, pure colonies on your isolation streak plates, congratulations!. Make sure that these potentially pure cultures have colonies that look like the original source colony and that all of the colonies look the same (they can be of different size). If you are having trouble obtaining a pure culture, consult with your instructor BEFORE LAB 5. We can't identify your isolate by 16s rRNA gene sequencing if it is not a pure culture.
Part A: Prepare Lysates from pure cultures of Bacteria of Interest
1. Each student will sequence DNA from 4-7 unique bacteria (up to 24 per team). Use well isolated colonies from your best plate (one that's unquestionably a pure culture) for each of your bacteria of interest. Consult with your partners to make sure that your team is identifying bacteria that are not unlikely to be of the same species and NOT likely to be mold or nonbacterial microbes.
2. Each students needs pcr tubes of your team color (0.2ml vol.). You will need one for each of your isolates. Wear new gloves when you take them out of the jar on the instructor bench and label each with a unique code to indicate each isolate you want to id. DO NOT CONTAMINATE THE INSIDE OF THE CAP with your skin flora. Use your sampling habitat id (site A, B, C, or D) and a unique set of numbers that your lab instructor will assign each student. Numbers will be found on the board.
3. Using your P20 micropipet, pipet 20μL of sterile water with 0.05% Non-idet P40 into each of the prelabeled pcr tubes. Nonidet-P40 is a detergent that keeps hydrophobic domains dispersed and, thus, helps to solubilize membranes. It is similar to Triton-x 100.
4. Touch a well isolated colony from a pure culture with a sterile and cooled inoculating needle or a sterile P10 micropipet tip (the tiny ones, not the P20 tips) and resuspend a barely visible amount of bacteria adhering to the tip by swishing the tip around in the appropriately labeled tube with water and detergent. Resist the urge to pick up too much cell material, but be aware that some of your isolates, particularly those dry, powdery Actinomycetes (like Steptomyces) are hard to get any cells to adhere. For those, it is ok to take a bit more. The tinest bit is enough, but make sure there is some part of the colony going into the lysate. Putting in too many cells can inhibit the pcr reaction and be as bad as putting in none.
5. Repeat for your other isolates-- into separate tubes.
6. Boil all samples for 5 minutes. This will lyse the cells and inactivate bacterial enzymes. You can boil in a heat block or in the thermal cycler-- if your instructor setup a program to boil and you used the small pcr tubes. Since she did and you did, we will boil the tubes in the thermal cycler today. Be careful when you remove the tubes from the thermal cycler. Point them away from you and ease the lids open while still covering the outside of the tops with your gloved fingers. Do this slowly and carefully with the opening pointed away from you. You don't want the caps to pop and make an aerosol of your bacteria and you don't want to lose your lysate.
Part B: PCR AMPLIFICATION of 16s rDNA from lysates prepared above
Note: All reagents for the pcr should be kept on ice and the master mix should be thawed on ice. Since DNA polymerase can function at room temp, we don't want the reaction to start until all the tubes are in the thermal cycler.
The components below have been aliquoted and prepared for you and are in pcr tubes of your team color. Label a pcr tube for each of your lysates with a fine point Sharpie on the top and side of the tube with the unique identifier for each bacterial isolate. We will set up one tube per lab as a neg control.
Setting up the PCR Mix
WEAR GLOVES AT ALL TIMES AND DON'T TOUCH THE INSIDE OF THE TUBE CAPS OR YOUR PIPET TIPS--Always use a new tip when going into anything in a pcr reaction. (Contamination is a significant problem in pcr)
Using a P2 or P10 and filter tips (remember that the P2 has two red decimal place volume indicators while the P10 only has 1 red decimal place indicator. MAKE SURE YOU HAVE DIALED IN THE CORRECT VOLUME!), add 4 microliters of your boiled lysate (containing the template DNA) to the prealiquoted 46 microliters of master mix (contains DNA polymerase, dNPTs, MgCl2, and buffers), primers and nuclease free water mixture described above (for a total volume of 50 μL) in clearly labeled pcr tubes of your team color. Make sure you label on both the top and sides of the tube. The tubes are tiny (holds only 0.2ml vol) and it is hard to write on them legibly but doing so is very important. You will have to use a unique identification code for your isolates and keep the key to the code in your lab notebook (also give a copy to your instructor).
Component TABLE
| Component | amt. in a 50 μl reaction |
Final Conc. |
|---|---|---|
| Purified DNAase free Water |
16 μl | |
| 2x Phusion Master Mix | 25 μl | 1x |
| 27F primer | 2.5 μl | 0.5 μMolar |
| 1492R primer | 2.5 μl | 0.5 μMolar |
| template DNA | 4 μl | 50-250ng optimum is 100ng of DNA/reaction |
Hold the tubes on ice until your instructor tells you the thermal cycler is ready to be loaded. Wipe the outside of the tubes to remove all ice and water before placing them in the thermal cycler.
For the negative control one person will add 4 microliters of water (in place of the template DNA). When you have mixed your DNA or water into the pcr mix by tapping VERY LIGHTLY or flicking to be sure that all reagents are mixed and not adhering to the tube wall, take your tubes to the thermal cycler when your instructor says it's ready. Keep them on ice until then, but wipe off the bottom of the tubes before putting them into the machine. Make a template key in your lab notebook as to where in the thermal cycler you put your tubes.
The thermal cycler program is, generally, similar for all pcr reactions, but the annealing temperature (melting) is dependent on the primer pair. When you design primers, the primer melting temp. can be calculated based on the GC content and other factors. Think about which would be harder to denature: GC pairs or AT pairs and why? For 27F and 1492R, a range of 45-55C is ok, although higher temp. may lead to increased specificity that excludes some organisms' DNA from being amplified.
The length of the fragment you are amplifying determines the extension time. A general rule of thumb is to use an extension time of 1kb per minute. Here, we amplify with primers designed for the 27th and 1492th positions in the 16s rDNA gene region. Therefore our fragment is expected to be about 1.5kb long, so we will use an extension time of 1.5 minutes per cycle.
Thermal Cycler Program:
3 step program
| Cycle Step | Temperature | Time | # of Cycles |
|---|---|---|---|
| Initial Denaturation | 98C | 30 sec. | 1 |
| Denaturation Annealing Extension |
98C 55C 72C |
10 sec 30 sec 30 sec |
35 |
| Final Extension | 72C 4C |
10 min Hold |
1 |
The pcr will run for 45min or so.
Part C: Clean Up of pcr product using Epoch BIoLabs GenCatch PCR CleanUp Kit
Before we can send your amplified 16s rRNA genes away for DNA sequencing, we must remove any left over primers, dNPTs, and other potential contaminants that might interfere with a sequencing reaction.
Notes before Starting:
Make sure 98% ethanol has been added to WN and WS Buffers before first time use (see bottles label for volume).
All centrifuge steps are carried out in a conventional tabletop microcentrifuge at roomtemperature.
Procedure
1. Measure 500 μl of Buffer PX using your P1000 and add ~100μL (do not need to be exact) of it to your thawed pcr product and the rest to a clean microfuge tube. Using your P200 set to 200 μL, remove all the pcrProduct/buffer mix in the pcr tube and add it to the PX buffer in the microfuge tube. Close the cap of the microfuge tube and mix.
2. Place a GenCatch™ spin column in a provided 2 ml collection tube.
3. Load all of the pcr product/bufferPX mixture created in step 1 to the spin column and centrifuge at 5000rpm for 60 sec.
4. Discard the flow-through in the collection tube. Place the spin column back into the same (now empty) collection tube.
(Collection tubes are re-used to reduce plastic waste.)
5. Wash the spin column once by adding 500 μL Buffer WN to the spin column and centrifuge for 60 sec at 5000rpm. Be careful to use WN buffer!!
6. Discard flow-through and place the spin column back in the same collection tube.
7. Wash the column by applying 500 μL of Buffer WS to the spin column. Note that WS Buffer is different than the buffer used in step 5. Centrifuge the column for 1 min. at 5000rpm. Discard the flow through.
8. Centrifuge the spin column in the same collection tube at full speed (~13,000rpm) for 3 more minutes to remove ethanol residue. It is crucially important to remove all ethanol residue; residual ethanol may inhibit subsequent enzymatic reactions!!! If you need to spin for an addition 3 min., it is ok to do so.
9. Place each spin column into a new, clean 1.5 ml microcentrifuge tube (not a collection tube. Do not proceed to the next step until there is NO smell of ethanol associated with the spin columns.
10. To elute DNA, add 50 μl of the Elution Buffer EB (10 mM Tris·Cl, pH 8.5) to the center of each spin column membrane. Let it stand for 2-4 minutes to allow it completely adsorb and then centrifuge the spin column in the microfuge tube for 2 min at highest speed, ~17,900 x g (13,000 rpm).
Keep your pcr product on ice until your instructor tells you that it's time to load the gel to determine the success of this amplification and clean-up.
IMPORTANT NOTES for using this kit: Ensure that the elution buffer is dispensed directly onto the spin column membrane for complete elution of bound DNA. The average eluate volume is 48 μl from 50 μl elution buffer volume.
Elution efficiency is dependent on pH. The maximum elution efficiency is achieved
between pH 7.0 and 8.5. Store DNA at –20°C as DNA may degrade in the absence of a buffering
agent.
Part D: Measuring the Concentration of DNA
There are two Nanodroppers in the BISC Equipment room, L308. Both the ThermoScientific NanoDrop 2000 and The NanoDrop ND-1000 Spectrophotometers measure DNA by taking Absorbance at A260nm. These spectrophotometers use only 1 microliter of sample and do not require cuvettes. The sample is held in place by fiber optic technology and surface tension that holds the sample in place between two optical surfaces that define the pathlength vertically and dynamically. Measurement can be assessed in a range of 2 to 3700nm/microliter dsDNA. These are expensive machines so make sure you follow the directions carefully and ask your instructor for guidance as needed.
More information is available from the manufacturer's website at: | http://www.nanodrop.com/HowItWorks.aspx

Activity: Using the Nanodroppers
1. Clean the upper and lower optical surfaces of the sample retension device by pipetting 2 microliters of clean deionized water onto the lower optical surface. Close the lever arm and tap it a few times to bathe the upper optical surface. Lift the lever arm and wipe off both optical surfaces with a Kimwipe.

2. Open the NanoDrop software from the Desktop of the computer and select the nucleic acids module.
3. Initialize the machine by placing 1 microliter of clean deionized water onto the lower optic surface, lower the lever arm, and select initialize from the NanoDrop software. Once initialization is complete (~10sec.), clean both optical surfaces with a Kimwipe.

4. Perform a blank measurement by loading 1 microliter of Elution Buffer (EB 10mM Tris) (Solution 6 in kit) and select Blank. Note that this blanking step may use something other than Tris depending on what your sample is dissolve in. Often the blank will be deionized water if you have concentrated your DNA sample already with the ethanol precipation and resolubilized it in water.
Note that as in traditional spectroscopy, the blank will be subtracted from subsequent measurements. If you want to determine the contribution of a specific buffer or diluent, measure the buffer first using distilled water as a blank. If the buffer does not contribute to the A 260nm reading, then deionized water will be fine to use as the blank. The water or buffer should always be measured to be sure that the instrument has been zeroed properly. The measurement of water or buffer should be zero or very close. All measurements are automatically normalized to 340nm.
5. Measure the nucleic acid sample by loading 1microliter of sample and selecting "measure". Record your DNA concentration. Once the measurement is complete. Clean both optical surfaces with a Kimwipe and the machine is ready for the next sample.
You should ensure that the appropriate constant (50 for dsDNA or 40 for RNA) has been chosen. The software automatically calculates the nucleic acid concentration. If the calculation is done by hand, the A260nm is represented as a 1cm path for convenience, even though 1-nm and 0.2nm paths are actually used during the measurement cycle.
Clean Up
When the last sample was been measured, clean the sampling device by repeating step 1.
Part E: Agarose Gel Electrophoresis of Clean PCR PRODUCT
To see if you successfully amplified the 16s rRNA gene and not anything else, you will "run a gel" on your cleaned pcr products. To run a gel means that we will perform an electrophoretic separation of the DNA fragments in your cleaned up pcr product, using 1/10 vol. of your pcr product applied to a 1% agarose gel stained with Sybr Safe DNA stain. Your instructor will photograph the gel, label it with your amplicon id from the template and post the gel photo to the Data folder in Resources in Sakai so you can evaluate your success at 16S rRNA gene amplification. You should see a single band of ~1.5kb indicating that the only dsDNA in your pcr product came from amplification of a ~1500bp gene fragment. Can you explain how we know the size of our amplified gene fragment?
Your agarose gel is made of 1.0% agarose solution (w/v) in 1x TGE buffer (10x=0.25 Tris, 1.9M Glycine, 13mM EDTA) with SybrSafe™ stain.
DNA is uniformly negatively charged and will,therefore, move toward the positive electrode. The separation is determined by the size or mass of the molecule or fragments of DNA.

Procedure for Agarose Gel Electrophoresis of PCR products
Load 1/10 of the total volume of pcr product (1 microliter minimum). In our case we should load 5 microliters.
You will put the 5 microliters of your pcr product as a spot on a small piece of parafilm and add 5 microliters of well mixed loading dye (0.25% XC, 30% glycerol, 0.1mg/ml RNAase). Mix by pipetting up and down before loading all 10 microliters into a lane of the 1% agarose gel (1% wt/vol in 1xTGE buffer with Sybr Safe DNA stain. Sybr Safe is a proprietary reagent from Invitrogen that cross-links a fluorescent compound to DNA and allows DNA to be visualized under UV light. It is prepared and used according to manufacturer's directions http://www.invitrogen.com). Apply your samples to the wells that you have signed up for on the gel template. Be sure to leave the first two lanes and the last lane empty for the 100bp ladder, the positive control and the negative water control.
Loading dye contains glycerol to keep our sample in the lane rather than floating away and will have one of 3 marker dyes (bromophenol blue, xylene cyanol, or
orange G) that facilitate estimation of DNA migration distance and optimization
of agarose gel run time. 1x TGE buffer is used in this electrophoretic separation (25mM Tris, 0.19M glycine, 1.3mM EDTA. The gel will be run at 120V for approximately 30 minutes.
How will you judge a successful amplification? How many fragments and of what size do you expect to see?
Make sure you give back the rest of your soil DNA isolate and the rest of the cleaned up pcr product to your instructor to freeze after the gel is loaded. Both are now in identical looking microfuge tubes with volume being the only visible difference. Make sure it is clear which is the pcr product and which is the genomic DNA isolate.
Make sure each of your pcr products is clearly labeled with your initials, lab section (Tues or Wed), soil sample identifier code letter, your unique code information for each bacterial isolate and the conc. of the DNA in ng/μL.
Your instructor will photograph and label the gel according to the template you have filled out and post the results to the data folder in Resources in Sakai.
Part F: Submitting Amplicons for Identification
Before your next lab, your instructors will prepare a 96 well plate of your pcr products that showed successful amplification of the 16s rRNA gene. We will send these samples to a commercial DNA sequencing facility. This important step of identification of bacterial members of your soil community is done for you but we want you to understand exactly what's going on in those automatic sequencing machines? Make sure you understand how chain termination (Sanger) sequencing works. We should have the sequencing data back by the time we are scheduled to learn how to analyze the data so we can find out, we hope, the identity of a few members of the bacteria in your soil community.
Cultured Bacterial Isolates: Examples of Competition and Co-operation in a Soil Community
Testing for Antibiotic Production
Start the Testing for Antibiotic Production.
Many microbes secrete antimicrobial compounds to help them compete with other microorganisms for habitat niche. Common antibiotic producers are the Actinomycetes (including Streptomycetes species)and many of the Bacillus species; although these are just a few among many, many antibiotic producing bacteria.
(This testing will take 3 weeks.)
Week 1:
Since the soil is the main source of microbes that supply the world's antibiotics, it's possible that you might discover the next great antimicrobial drug and get very rich by selling the patent for your discovery to a drug company. (Remember that the discovery of penicillin was completely accidental.) In order to find out if any of your isolates are potential antibiotic producers, we will grow your isolates for 1 week on NA before we add the control bacteria or other isolates that may be sensitive to the antibiotic produced and secreted into the medium by the first isolate. You should test all of your isolates for antibiotic production. Be espectially sure to test any isolates that grew originally on glycerol yeast extract medium, because those bacteria are likely to be spore formers. Endospore formers are often antibiotic producers.
1) To begin the antibiotic testing protocol, you will use the fresh broth culture of each of your isolates that you prepared prior to lab.
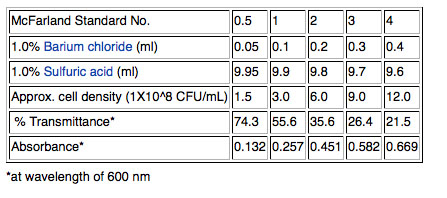
2) Although cell density can be measured more accurately using a spectrophotometer to measure optical density (OD) at 600nm, we will use a quicker method that will work well enough for our purposes. Pipet one ml of the broth culture into a sterile glass tube and adjust the concentration of the cells by matching by eye the "cloudiness" (turbidity) of the cells in the glass tube to a standard provided by your instructor. We will use a McFarland 0.5 standard; the 0.5 refers to the approximate concentration of organisms in solutions which is 1.5X108 cfu/mL for the 0.5 standard. Other common standards are shown in the table below.
3) Add bacteria from a colony of the same isolate grown on solid medium to concentrate the cells in your tube if your culture seems too dilute or add more sterile Nutrient broth to dilute the culture aliquot if it is already cloudier than the standard. The ending volume is irrelevant. Vortex to mix.
4) Using a sterile swab, dip the swab in the concentration adjusted culture tube and make an inoculation (as shown below) down the middle of a plate of nutrient agar.
5) Make a plate for each isolate to be tested. Label the plates carefully and incubate them for 1 week at RT.
Characterization of Soil Bacteria: Maintaining Pure Cultures of Isolates
Continue to subculture your pure isolates to fresh NA plates each week (isolation streak a well isolated colony onto a fresh plate), in the next lab you will make a bacterial smear and do a Gram stain and start other tests to provide examples of co-operative and competitive community behavior among the members of your soil community.
Preparation for Next Lab
Tests of your isolates will continue next week. You will use the sub-cultures made today or their antecedents or descendants. Depending on the test, you may need a fresh liquid broth culture, or an isolation streak plate culture on NA. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. The number of hours it takes from inoculation until a bacterial culture moves from log to stationary or death phase depends on its generation time, the concentration of the inoculum, and other factors. If you have a reasonably fast growing bacterial strain, you should make a subculture into Nutrient broth solid and liquid medium about 24-48 hours before you are to set up a new test. If you have a particularly slow grower, those cultures need to be set up earlier than that. Keep track of how fast each of your soil bacterial isolates grows and plan accordingly.
Reference Information
Your most important resource for looking up information about your isolates will be the reference manuals: THE PROKARYOTES and Bergey's Manual. Wellesley College has these valuable reference books available in electronic form. Link to the electronic edition of | The Prokaryotesthrough Springer ebooks.
Link to the electronic edition of | Bergey's Manualsthrough Springer ebooks.
Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests you will perform or to look up the usual characteristics of the isolates that are identified by 16s rRNA gene sequencing.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
M&M: Compose a draft of your Materials and Methods section of your final paper with the following general sections:
1) Obtaining a soil sample & making a soil extract
2) Enummeration of microbes in a soil community;
a) Cuture-Dependent Colony Count
b) Culture-Independent Direct Count
3) Identification of bacteria in a soil community by 16S rRNA gene sequencing;
a) Isolating Bacteria of Selected Groups to Pure Culture
b) Amplifying the 16srRNA gene by colony pcr & preparation for Gene Sequencing
4) Soil Microbial Co-operation & Competition:
a) Culture-Dependent Microbial Community Assessment of Functional Metabolic Diversity
1) Carbon source utilization pattern and diversity
b) Co-operation and Competition among Cultured Bacterial isolates from a Soil Community
1) Prevalence of Starch, Cellulose, and Phosphate digesters or processors
More information can be found at Lab 5 Assignment: Materials & Methods
In Lab 6-8, you will doing most of the assessment of your isolates' through a battery of tests and special stains, a few of which require some preparatory work. Make sure you set up a fresh broth culture of appropriate media for each isolate a few days before your next lab and have a fresh streak plate of each isolate ready for next week's tests. Familiarize yourself with the tests and stains you will perform. Make sure you have outlined the protocols in your lab notebook and started any necessary cultures on appropriate medium so that they will be ready to use in lab at the appropriate time: