BISC209: Lab4: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| Line 9: | Line 9: | ||
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends. <BR><BR> | phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends. <BR><BR> | ||
[[Image: | [[Image:toposhema.jpg]] | ||
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit should work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form ''E. coli'' that we will use for separating the amplified 16s rDNA from our soil flora.<BR><BR> | We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit should work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form ''E. coli'' that we will use for separating the amplified 16s rDNA from our soil flora.<BR><BR> | ||
Revision as of 11:03, 22 December 2009
LAB 4: Con't. Project: Soil Microbial Communities & Diversity
Your instructor will return your frozen pcr products containing amplified fragments of 16s rDNA from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rDNA fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of Escherichia coli bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin.
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends.
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit should work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form E. coli that we will use for separating the amplified 16s rDNA from our soil flora.
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death) encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. (Cool technology; yes!?)

Protocol for using Zero Blunt TOPO PCR Clonging Kit with One Shot TOP 10 Chemically Competent E. coli
PCR cloning requires three steps.
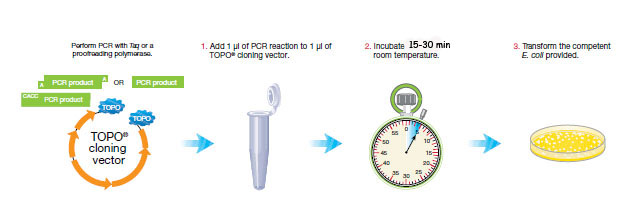
1. Add 1 μl of PCR reaction to 1 μl of TOPO® cloning vector.
2. Incubate 5 min at room temperature.
3. Transform Oneshot Top10 competent E. coli.
Protocol for Transforming TOPO Competent E. coli
Genotype of OneShot TOP10 Competent Cells: F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG
General Handling: Be extremely gentle when working with competent cells. Competent cells are highly sensitive to changes in temperature or mechanical lysis caused by
pipetting. Transformation should be started immediately following the thawing
of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting.
Transforming One Shot® Competent Cells
Introduction: Once you have performed the TOPO® Cloning reaction, you will transform your pCR®-Blunt II-TOPO® construct into competent E. coli provided with your kit.
You will need to gather:
In addition to general microbiological supplies (e.g. petri dish with ethanol, glass spreader or sterile glass beads), you will need the following reagents and equipment.
• TOPO® Cloning reaction from Performing the TOPO® Cloning Reaction
• S.O.C. medium (included with the kit)
• 42°C water bath
• LB plates containing 50 μg/ml kanamycin or Low Salt LB plates containing
25 μg/ml Zeocin™ (use two plates per transformation; see recipes below)
• 37°C shaking and non-shaking incubators
Preparing forTransformation
For each transformation, you will need one vial of competent cells and two
selective plates.
• Equilibrate a water bath to 42°C
• Bring the vial of S.O.C. medium to room temperature.
• Warm LB plates containing 50 μg/ml kanamycin or 25 μg/ml Zeocin™ at 37°C
for 30 minutes.
• Thaw on ice 1 vial of One Shot® cells for each transformation.
NOTE:If you are transforming One Shot® Mach1™-T1R Chemically Competent E. coli, it
is essential that selective plates are prewarmed to 37° prior to spreading for
optimal growth of cells.
1. Add 2 μl of the TOPO® Cloning reaction from Performing the TOPO®
Cloning Reaction into a vial of One Shot® Chemically
Competent E. coli and mix gently. Do not mix by pipetting up and down!
2. Incubate on ice for 5 to 30 minutes.
Note: Longer incubations on ice do not seem to have any affect on transformation
efficiency. The length of the incubation is at the user’s discretion.
3. Heat-shock the cells for 30 seconds at 42°C without shaking.
4. Immediately transfer the tubes to ice.
5. Add 250 μl of room temperature S.O.C. medium.
6. Cap the tube tightly and shake the tube horizontally (200 rpm) at 37°C for
1 hour.
7. Spread 10-50 μl from each transformation on a prewarmed selective plate
and incubate overnight at 37°C. To ensure even spreading of small volumes,
add 20 μl of S.O.C. medium. We recommend that you plate two different
volumes to ensure that at least one plate will have well-spaced colonies.
Incubate plates over night at 37°C.
8. An efficient TOPO® Cloning reaction will produce several hundred
colonies.
Media Recipes
1
