BISC209: Lab4: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| Line 24: | Line 24: | ||
2. Incubate 5 min at room temperature.<BR> | 2. Incubate 5 min at room temperature.<BR> | ||
3. Transform Oneshot Top10 competent ''E. coli''.<br><BR> | 3. Transform Oneshot Top10 competent ''E. coli''.<br><BR> | ||
Since we are TOPO® Cloning large pool of PCR products, we need to maximize transformants to obtain the maximum number of clones.<BR> | |||
'''To increase the number of colonies:'''<BR> | |||
Addition of the Dilute Salt Solution in the TOPO® Cloning Reaction brings the | |||
final concentration of NaCl and MgCl2 in the TOPO® Cloning reaction to 50 mM | |||
and 2.5 mM, respectively.<BR> | |||
Incubate the salt-supplemented TOPO® Cloning reaction for 20 to 30 minutes | |||
instead of 5 minutes.<BR> | |||
''Increasing the incubation time of the salt-supplemented TOPO® Cloning | |||
reaction allows more molecules to ligate, increasing the transformation | |||
efficiency. Addition of salt appears to prevent topoisomerase from rebinding | |||
and nicking the DNA after it has ligated the PCR product and dissociated from | |||
the DNA.'' | |||
<BR><BR> | |||
==Protocol for Transforming TOPO Competent ''E. coli''== | ==Protocol for Transforming TOPO Competent ''E. coli''== | ||
Revision as of 15:43, 22 December 2009
LAB 4: Con't. Project: Soil Microbial Communities & Diversity
Your instructor will return your frozen pcr products containing amplified fragments of 16s rDNA from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rDNA fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of Escherichia coli bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin.
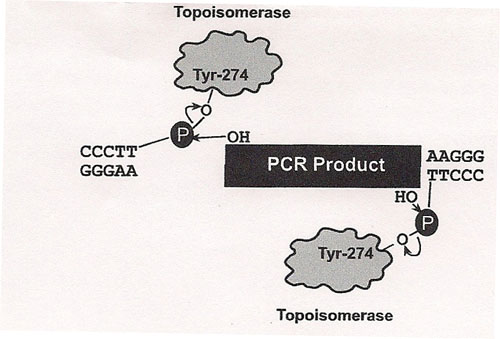
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends.
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit should work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form E. coli that we will use for separating the amplified 16s rDNA from our soil flora.
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death) encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. (Cool technology; yes!?)
Protocol for using Zero Blunt TOPO PCR Clonging Kit with One Shot TOP 10 Chemically Competent E. coli
PCR cloning requires three steps.
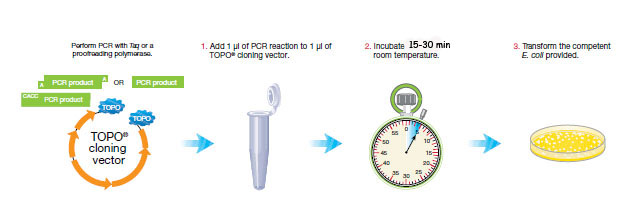
1. Add 1 μl of PCR reaction to 1 μl of TOPO® cloning vector.
2. Incubate 5 min at room temperature.
3. Transform Oneshot Top10 competent E. coli.
Since we are TOPO® Cloning large pool of PCR products, we need to maximize transformants to obtain the maximum number of clones.
To increase the number of colonies:
Addition of the Dilute Salt Solution in the TOPO® Cloning Reaction brings the
final concentration of NaCl and MgCl2 in the TOPO® Cloning reaction to 50 mM
and 2.5 mM, respectively.
Incubate the salt-supplemented TOPO® Cloning reaction for 20 to 30 minutes
instead of 5 minutes.
Increasing the incubation time of the salt-supplemented TOPO® Cloning
reaction allows more molecules to ligate, increasing the transformation
efficiency. Addition of salt appears to prevent topoisomerase from rebinding
and nicking the DNA after it has ligated the PCR product and dissociated from
the DNA.
Protocol for Transforming TOPO Competent E. coli
Genotype of OneShot TOP10 Competent Cells: F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG
General Handling: Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting.
Transforming One Shot® Competent Cells
Introduction: Once you have performed the TOPO® Cloning reaction, you will transform your pCR®-Blunt II-TOPO® construct into TOPO10 competent E. coli provided with your kit.
You will need to gather:
In addition to general microbiological supplies (e.g. petri dish with ethanol, glass spreader or sterile glass beads), you will need the following reagents and equipment.
• TOPO® Cloning reaction from Performing the TOPO® Cloning Reaction
• S.O.C. medium at room temp.(included with the kit)
• 42°C water bath
• LB plates containing 50 μg/ml kanamycinBR>
• 37°C shaking and non-shaking incubators
Preparing forTransformation
For each transformation, you will need one vial of competent cells and two
selective plates.
• Equilibrate a water bath to 42°C
• Bring the vial of S.O.C. medium to room temperature.
• Warm LB plates containing 50 μg/ml kanamycin at 37°C
for 30 minutes.
• Thaw on ice 1 vial of One Shot® cells for each transformation.
Transformation Protocol
1. Add 2 μl of the TOPO® Cloning reaction when it is completed into a vial of One Shot® Chemically Competent E. coli and mix gently by swirling. Do not mix by pipetting up and down!
2. Incubate on ice for 30 minutes.
Note: Longer incubations on ice do not seem to have any affect on transformation
efficiency. The length of the incubation is at the user’s discretion.
3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).
4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .
5. Add 250 μl of room temperature S.O.C. medium.
6. Cap the tube tightly and put it in a 250 ml shake the tube horizontally (200 rpm) at 37°C for
1 hour. Slightly dehydrate 2 LB + kan plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then places the plates in the 37C incubator to prewarm.
7. Using a micropipet, pipet 10-50 μl (your instructor will advise you on the exact volume) from each transformation in the center of a prewarmed selective plate and add 20 μl of S.O.C. medium to the transformation mix on the plate. This dilutes the cells and makes spreading of small volumes easier. Using your alcohol flamed glass spreader or sterile glass beads, carefully spread your cells over the entire surface of the plate.
8. Repeat step 7 using a different volume of cells. It is recommended that you plate at least two different volumes to ensure that at least one plate will have well-spaced colonies.
9. Incubate all plates upside down overnight at 37°C. Remember to label them with all the appropriate information: your initials, lab section, date, your soil sample id, the type of medium, and the id of the cells. .
10. An efficient TOPO® Cloning reaction will produce several hundred
colonies.
Media Recipes
S.O.C. Medium
(may be stored at +4°C or
room temperature)
2% Tryptone
0.5% Yeast Extract
10 mM NaCl
2.5 mM KCl
10 mM MgCl2
10 mM MgSO4
20 mM glucose
LB agar plates
1. Prepare LB medium as above, but add 15 g/L agar before autoclaving.
2. Autoclave on liquid cycle for 20 minutes at 15 psi.
3. After autoclaving, cool to ~55°C, add antibiotic (50 μg/ml of kanamycin),
and pour into 10 cm plates.
4. Let harden, then invert and store at +4°C, in the dark.