BISC209: Lab4: Difference between revisions
| Line 109: | Line 109: | ||
Hopefully the colonies from all four of the second set of isolation streak plates prepared in lab 3 grew, look isolated, look like the original source colony, and are pure. If you are having trouble at this phase consult with the instructor. You may need to return to the original plates, to try isolation streaking again, or borrow an organism from someone else. <BR><BR> | Hopefully the colonies from all four of the second set of isolation streak plates prepared in lab 3 grew, look isolated, look like the original source colony, and are pure. If you are having trouble at this phase consult with the instructor. You may need to return to the original plates, to try isolation streaking again, or borrow an organism from someone else. <BR><BR> | ||
Each student should successfully isolate 3-4 different organisms. <BR><BR> | Each student should successfully isolate 3-4 different organisms. <BR><BR> | ||
Transfer about 1/8 of each colony to two stock slants (continue to use the media on which the colony was isolated) and 1/8 of each to a new isolation streak plate. Grow the slants and plates for the appropriate number of days and then store these stocks in the designated area of the cold room. Keep one of the 2 slants in the cold room untouched and use the other next week for additional inoculations. <BR><BR> | Transfer about 1/8 of each colony to two stock slants (continue to use the media on which the colony was isolated) and 1/8 of each to a new isolation streak plate [[BISC209: Streaking for Isolation | Streaking for Isolation ]]. Grow the slants and plates for the appropriate number of days and then store these stocks in the designated area of the cold room. Keep one of the 2 slants in the cold room untouched and use the other slant next week for additional inoculations. The isolation streak plate may also be useful next week either for inoculating or as a backup if your Gram Stain doesn't look pure.<BR><BR> | ||
'''Activity C-2''' Gram Stain <BR><BR> | '''Activity C-2''' Gram Stain <BR><BR> | ||
Use 1/8 to 1/4 of each selected isolated colony to perform a [[BISC209: The Gram Stain | Gram Stain]] to see if all the cells appear identical microscopically and to determine the Gram reaction. The Gram reaction and organism morphology are the first step in deciding what tests you will perform to continue with the identification. Put 2-3 smears on one slide and stain them simultaneously to save time. Please remind yourself of proper use of the microscope and oil immersion [[BISC209: Microscopy | Microscopy: Care and Use of the Compound Brightfield Microscope]]<BR><BR> | Use 1/8 to 1/4 of each selected isolated colony to perform a [[BISC209: The Gram Stain | Gram Stain]] to see if all the cells appear identical microscopically and to determine the Gram reaction. The Gram reaction and organism morphology are the first step in deciding what tests you will perform to continue with the identification. Put 2-3 smears on one slide and stain them simultaneously to save time. Please remind yourself of proper use of the microscope and oil immersion [[BISC209: Microscopy | Microscopy: Care and Use of the Compound Brightfield Microscope]]<BR> <BR> | ||
'''Activity C-3: Spot inoculation technique on selective media.'''<BR><BR> | '''Activity C-3: Spot inoculation technique on selective media.'''<BR><BR> | ||
Revision as of 12:45, 10 January 2010
LAB 4: Con't. Project: Soil Microbial Communities & Diversity
Your instructor will return your frozen pcr products containing amplified fragments of 16s rDNA from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rDNA fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of Escherichia coli bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin.
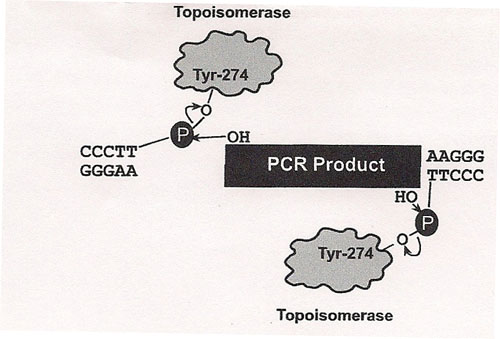
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends.
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit should work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form E. coli that we will use for separating the amplified 16s rDNA from our soil flora.
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death) encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. (Cool technology!)
Part A: Using Zero Blunt TOPO PCR Cloning Kit with One Shot TOP 10 Chemically Competent E. coli
PCR cloning requires three steps.
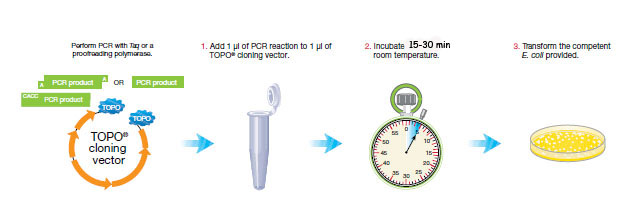
Procedure:
1. Add 1 μl of PCR reaction to 1 μl of TOPO® cloning vector.
2. Incubate 5 min at room temperature.
3. Transform Oneshot Top10 competent E. coli.
Since we are TOPO® Cloning large pool of PCR products, we need to maximize transformants to obtain the maximum number of clones.
To increase the number of colonies:
Addition of the Dilute Salt Solution in the TOPO® Cloning Reaction brings the
final concentration of NaCl and MgCl2 in the TOPO® Cloning reaction to 50 mM
and 2.5 mM, respectively.
Incubate the salt-supplemented TOPO® Cloning reaction for 20 to 30 minutes
instead of 5 minutes.
Increasing the incubation time of the salt-supplemented TOPO® Cloning
reaction allows more molecules to ligate, increasing the transformation
efficiency. Addition of salt appears to prevent topoisomerase from rebinding
and nicking the DNA after it has ligated the PCR product and dissociated from
the DNA.
Part B Transforming TOPO Competent E. coli
Genotype of OneShot TOP10 Competent Cells: F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG
General Handling: Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting.
Transforming One Shot® Competent Cells
Introduction: Once you have performed the TOPO® Cloning reaction, you will transform your pCR®-Blunt II-TOPO® construct into TOPO10 competent E. coli provided with your kit.
You will need to gather:
In addition to general microbiological supplies (e.g. petri dish with ethanol, glass spreader or sterile glass beads), you will need the following reagents and equipment.
• TOPO® Cloning reaction from Performing the TOPO® Cloning Reaction
• S.O.C. medium at room temp.(included with the kit)
• 42°C water bath
• LB plates containing 50 μg/ml kanamycin
• 37°C shaking and non-shaking incubators
Preparing for Transformation
For each transformation, you will need one vial of competent cells and two
selective plates.
• Equilibrate a water bath to 42°C
• Bring the vial of S.O.C. medium to room temperature.
• Warm LB plates containing 50 μg/ml kanamycin at 37°C
for 30 minutes.
• Thaw on ice 1 vial of One Shot® cells for each transformation.
Transformation Procedure
1. Add 2 μl of the TOPO® Cloning reaction when it is completed into a vial of One Shot® Chemically Competent E. coli and mix gently by swirling. Do not mix by pipetting up and down!
2. Incubate on ice for 30 minutes.
Note: Longer incubations on ice do not seem to have any affect on transformation
efficiency. The length of the incubation is at the user’s discretion.
3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).
4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .
5. Add 250 μl of room temperature S.O.C. medium.
6. Cap the tube tightly and put it in a 250 ml Erlenmyer flask and shake the tube horizontally (200 rpm) at 37°C for
1 hour. Slightly dehydrate 2 LB + kan plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then places the plates in the 37C incubator to prewarm.
7. Using a micropipet, pipet 10-50 μl (your instructor will advise you on the exact volume) from each transformation in the center of a prewarmed selective plate and add 20 μl of S.O.C. medium to the transformation mix on the plate. This dilutes the cells and makes spreading of small volumes easier. Using your alcohol flamed glass spreader or sterile glass beads, carefully spread your cells over the entire surface of the plate.
8. Repeat step 7 using a different volume of cells. It is recommended that you plate at least two different volumes to ensure that at least one plate will have well-spaced colonies.
9. Incubate all plates upside down overnight at 37°C. Remember to label them with all the appropriate information: your initials, lab section, date, your soil sample id, the type of medium, and the id of the cells. .
10. An efficient TOPO® Cloning reaction will produce several hundred
colonies.
Transformation Media Recipes
S.O.C. Medium
(may be stored at +4°C or
room temperature)
2% Tryptone
0.5% Yeast Extract
10 mM NaCl
2.5 mM KCl
10 mM MgCl2
10 mM MgSO4
20 mM glucose
LB agar plates
1. Prepare LB medium as above, but add 15 g/L agar before autoclaving.
2. Autoclave on liquid cycle for 20 minutes at 15 psi.
3. After autoclaving, cool to ~55°C, add antibiotic (50 μg/ml of kanamycin),
and pour into 10 cm plates.
4. Let harden, then invert and store at +4°C, in the dark.
Part C: Isolating Culturable Bacteria from soil continued
Activity C-1: Pure cultures of (4) isolates
Hopefully the colonies from all four of the second set of isolation streak plates prepared in lab 3 grew, look isolated, look like the original source colony, and are pure. If you are having trouble at this phase consult with the instructor. You may need to return to the original plates, to try isolation streaking again, or borrow an organism from someone else.
Each student should successfully isolate 3-4 different organisms.
Transfer about 1/8 of each colony to two stock slants (continue to use the media on which the colony was isolated) and 1/8 of each to a new isolation streak plate Streaking for Isolation . Grow the slants and plates for the appropriate number of days and then store these stocks in the designated area of the cold room. Keep one of the 2 slants in the cold room untouched and use the other slant next week for additional inoculations. The isolation streak plate may also be useful next week either for inoculating or as a backup if your Gram Stain doesn't look pure.
Activity C-2 Gram Stain
Use 1/8 to 1/4 of each selected isolated colony to perform a Gram Stain to see if all the cells appear identical microscopically and to determine the Gram reaction. The Gram reaction and organism morphology are the first step in deciding what tests you will perform to continue with the identification. Put 2-3 smears on one slide and stain them simultaneously to save time. Please remind yourself of proper use of the microscope and oil immersion Microscopy: Care and Use of the Compound Brightfield Microscope
Activity C-3: Spot inoculation technique on selective media.
This week, you only have about 1/8 to 1/4 of the colony for each organism left. You will "confirm" the Gram reaction by preparing plates on either EMB and PEA media see Use of Selective, Differential, Enrichment Media. On the bottom of each dish, divide the plate into 4 quadrants with a marker and organize a labeling system in your lab notebook and on the plate so you can easily identify the source organism and quadrant next week. You will spot inoculate the middle of each quadrant with a small amount of the remaining growth from the selected colony. If you do not have enough growth to do this today, label the plates, and place them in your section of the cold room for use next lab.


The inoculation of biochemical test media in this lab will occur over the next few weeks using your stock cultures. Sometimes you will need a newly grown agar slant, liquid broth or isolation streak plate. You will need to read ahead and prepare these "stocks" a few days or 1 week prior to the lab in which it will be needed. The timing depends on each different organism so keep track of this on your own in you lab notebook. During this time it is essential that your isolates remain fresh and uncontaminated. If your plates or slants appear old or contaminated, ask for fresh media, and prepare a new plate or slant always using the media on which you isolated your organism. It is good practice to re-streak your strains regularly, so that they remain fresh.
Remember: Each time you use a stock slant to inoculate more media, you should first make a fresh stock slant, grow it the appropriate amount of time, and then store it in the cold room until needed.
Classification of Unknown Isolates
Your most important resource for identification will be the on-line reference: THE PROKARYOTES and Bergey's Manual. Use these in conjunction with the select tests we have available in the lab. It is unlikely we will be able to provide ALL the tests you might wish to perform, but it is likely that you will be able to identify your organisms to at least Genera and combined with the Genomic test you will perform later in the semester, you will identify at least one organism to species. You will also investigate the role your organism might play in the soil ecosystem. The biochemical bases and interpretation of the results of the variety of tests available for this lab are explained on the appropriate sections of the protocol page in this wiki.