BISC209: Lab4: Difference between revisions
| Line 23: | Line 23: | ||
Procedure:<BR> | Procedure:<BR> | ||
1. Add 1 μl of PCR reaction to 1 μl of TOPO® cloning vector.<BR> | 1. Add 1 μl of PCR reaction to 1 μl of TOPO® cloning vector.<BR> | ||
2. Incubate 15 min at room temperature.<BR> | 2. Add 1 μL of salt solution | ||
3. Add 3 μL of purified water | |||
4. Incubate 15 min at room temperature.<BR> | |||
3. Transform Oneshot Top10 competent ''E. coli''.<br><BR> | 3. Transform Oneshot Top10 competent ''E. coli''.<br><BR> | ||
Revision as of 07:52, 15 January 2010
LAB 4: Con't. Project: Soil Microbial Communities & Diversity
Your instructor will return your frozen pcr products containing amplified fragments of 16s rDNA from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rDNA fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of Escherichia coli bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin.
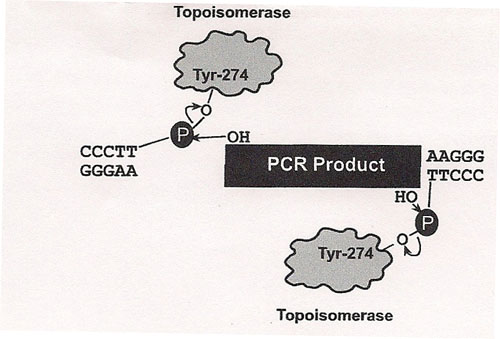
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends.
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit should work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form E. coli that we will use for separating the amplified 16s rDNA from our soil flora.
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death) encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. (Cool technology!)
Part A: Using Zero Blunt TOPO PCR Cloning Kit with One Shot TOP 10 Chemically Competent E. coli
PCR cloning requires three steps.
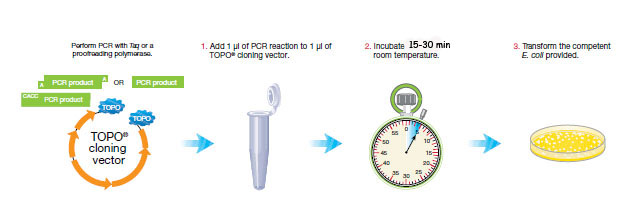
Procedure:
1. Add 1 μl of PCR reaction to 1 μl of TOPO® cloning vector.
2. Add 1 μL of salt solution
3. Add 3 μL of purified water
4. Incubate 15 min at room temperature.
3. Transform Oneshot Top10 competent E. coli.
Since we are TOPO® Cloning large pool of PCR products, we need to maximize transformants to obtain the maximum number of clones.
To increase the number of colonies:
Addition of the Dilute Salt Solution in the TOPO® Cloning Reaction brings the
final concentration of NaCl and MgCl2 in the TOPO® Cloning reaction to 50 mM
and 2.5 mM, respectively.
Incubate the salt-supplemented TOPO® Cloning reaction for 20 to 30 minutes
instead of 5 minutes.
Increasing the incubation time of the salt-supplemented TOPO® Cloning
reaction allows more molecules to ligate, increasing the transformation
efficiency. Addition of salt appears to prevent topoisomerase from rebinding
and nicking the DNA after it has ligated the PCR product and dissociated from
the DNA.
Part B Transforming TOPO Competent E. coli
Genotype of OneShot TOP10 Competent Cells: F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG
General Handling: Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting.
Transforming One Shot® Competent Cells
Introduction: Once you have performed the TOPO® Cloning reaction, you will transform your pCR®-Blunt II-TOPO® construct into TOPO10 competent E. coli provided with your kit.
You will need to gather:
In addition to general microbiological supplies (e.g. petri dish with ethanol, glass spreader or sterile glass beads), you will need the following reagents and equipment.
• TOPO® Cloning reaction from Performing the TOPO® Cloning Reaction
• S.O.C. medium at room temp.(included with the kit)
• 42°C water bath
• LB plates containing 50 μg/ml kanamycin
• 37°C shaking and non-shaking incubators
Preparing for Transformation
For each transformation, you will need one vial of competent cells and two
selective plates.
• Equilibrate a water bath to 42°C
• Bring the vial of S.O.C. medium to room temperature.
• Warm LB plates containing 50 μg/ml kanamycin at 37°C
for 30 minutes.
• Thaw on ice 1 vial of One Shot® cells for each transformation.
Transformation Procedure
1. Add 2 μl of the TOPO® Cloning reaction when it is completed into a vial of One Shot® Chemically Competent E. coli and mix gently by swirling. Do not mix by pipetting up and down!
2. Incubate on ice for 30 minutes.
Note: Longer incubations on ice do not seem to have any affect on transformation
efficiency. The length of the incubation is at the user’s discretion.
3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).
4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .
5. Add 250 μl of room temperature S.O.C. medium.
6. Cap the tube tightly and put it in a 250 ml Erlenmyer flask and shake the tube horizontally (200 rpm) at 37°C for
1 hour. Slightly dehydrate 2 LB + kan plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then places the plates in the 37C incubator to prewarm.
7. Using a micropipet, pipet 10-50 μl (your instructor will advise you on the exact volume) from each transformation in the center of a prewarmed selective plate and add 20 μl of S.O.C. medium to the transformation mix on the plate. This dilutes the cells and makes spreading of small volumes easier. Using your alcohol flamed glass spreader or sterile glass beads, carefully spread your cells over the entire surface of the plate.
8. Repeat step 7 using a different volume of cells. It is recommended that you plate at least two different volumes to ensure that at least one plate will have well-spaced colonies.
9. Incubate all plates upside down overnight at 37°C. Remember to label them with all the appropriate information: your initials, lab section, date, your soil sample id, the type of medium, and the id of the cells. .
10. An efficient TOPO® Cloning reaction will produce several hundred
colonies.
Transformation Media Recipes
S.O.C. Medium
(may be stored at +4°C or
room temperature)
2% Tryptone
0.5% Yeast Extract
10 mM NaCl
2.5 mM KCl
10 mM MgCl2
10 mM MgSO4
20 mM glucose
LB agar plates
1. Prepare LB medium as above, but add 15 g/L agar before autoclaving.
2. Autoclave on liquid cycle for 20 minutes at 15 psi.
3. After autoclaving, cool to ~55°C, add antibiotic (50 μg/ml of kanamycin),
and pour into 10 cm plates.
4. Let harden, then invert and store at +4°C, in the dark.
Part C: Isolating Culturable Bacteria from soil continued
Activity C-1: Pure cultures of (4) isolates
We hope that you have well isolated, pur colonies from each of your isolation streak plates prepared in LABS 2 and 3. If so, congratulations!. Make sure that these potentially pure cultures have colonies that look like the original source colony and that all of the colonies look the same (they can be of different size). If you are having trouble with obtaining well isolated colonies, consult with your instructor. You may need to return to the original plates, to try isolation streaking again.
Each pair of students should try to successfully isolate bacteria from as many DIFFERENT types of enrichment media as possible. Your goal is that each person in a team of 4 students sampling from the same habitat will isolate and identify 4 different soil bacteria each from as many different groups as possible. Coordinate with your team members to attempt to isolate colonies that look different from each other and that include at least one or two potential antibiotic producers, cellulolytic bacteria, ammonia reducers, and other different interesting types of bacteria per team. It would be great if each team identified 16 different bacteria in your soil habitat and at least one from each of the groups that we have enrichment media to find. That may not be possible but it will make your poster presentation at the end of this project more interesting if you all don't culture and identify the same or the same type of bacteria.
Making STOCK CULTURES
If you are at the stage of having pure cultures continue with the following Activities--if not wait until you do have single colonies in a pure culture to do these procedures.
Aseptically transfer ~1/8 of a colony from each of your pure cultures to two stock slants. Continue to use the enrichment or general purpose media on which the colony was isolated and grew well. Follow the Aspectic Transfer protocol .
Aseptically transfer another 1/8 of a colony of each pure culture to a new agar plate of the appropriate soid medium (the same type from which to take this colony). See Streaking for Isolation .
Grow these cultures (slants and plates)at the appropriate temperature until you get more mature, healthy, well isolated colonies and then store these stocks in the cold room in the designated place.
Always keep one stock slant in the cold room untouched.
Use the other slant for the next inoculation. Every time you use a stock slant, replace it by starting another so there is always one untouched one to use and one untouched one in the cold room in case the other is contaminated.
Also keep your isolation streak plate (sealed with parafilm) in the refrigerator as a potentially useful backup to your slant.
Activity C-2: Gram Stain
Use 1/8 of a well-isolated colony of each bacterial soil isolate to make a smear slide, Smear Slide Preparation, and to perform a Gram Stain. The Gram stain provides information about the cell wall composition, and observing stained cells allows you to assess this bacterial species' shape and characteristic arrangement. You will also be able to determine whether or not all the cells appear identical. If they don't, you may have a pleomorphic organism or your isolate may not be as pure as you thought. The Gram reaction and the morphologic characteristics that you can assess from this procedure are sometimes crucial in identifying the genera of bacteria that you have isolated.
Prepare smears by placing 2-3 smears on one slide and stain them simultaneously to save time, as described in Smear Slide Preparation
. Carefully perform the Gram Stain Please follow scrupulously the instructions on proper use of the microscope, particularly when using the oil immersion objective. Those instructions are found at: Microscopy: Care and Use of the Compound Brightfield Microscope
Activity C-3: Performing a Spot Inoculation Technique on Selective Media.
Since you only have about 1/8 to 1/4 of the original colonies used in the activities above for each organism left, be judicioius when you "confirm" the Gram reaction by inoculating solid selective and differential media or either, but not both, EMB and PEA media. Consult Use of Selective, Differential, Enrichment Mediato determine which of these two selective and differential media you should use. On the bottom of each plate, divide it into 4 quadrants with a marker and organize a labeling system in your lab notebook and on the plate so you can easily identify where you placed each of your soil isolates. You will spot inoculate the middle of each quadrant by streaking a tiny amount of the remaining growth from the selected colony in single zig-zag over the quadrant. See the illustration below. If you do not have enough of the colony left to do this today, label the plates, and place them in your section of the cold room for use next lab when your inoculated stock subcultures have restored your supply of bacteria to test. Remember that bacteria reproduce asexually by binary fission so that if a colony comes from a single cell and you only use one colony or its descendents for all of your tests, you have used a genetically identical population (excluding spontaneous mutations) of cells for all of your tests over the semester.


The inoculation of biochemical test media will occur over the next few weeks. You will use the stock cultures that you have started this week or their descendents. Sometimes you will need a newly grown agar slant culture, liquid broth culture or isolation streak plate culture. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. The time that cultures take to mature to readiness depends on each different organism, so keep track of how fast each of your soil bacteria grow, on which media,and at what temperature. Since this is an investigative lab that is largely designed and executed individually and with your team, success will require considerable planning and organization as well as copious notetaking.
It is essential that your stock slant cultures remain fresh and uncontaminated. Don't forget to replace a streak plate or slant frequently, using the media on which you were able isolated that type of bacteria.
Remember: Each time you use a stock slant to inoculate more media, you should first make a fresh stock slant, grow it the appropriate amount of time, and then store it in the cold room until needed.
Classification of Unknown Isolates
Your most important resource for identification will be the reference manuals: THE PROKARYOTES and Bergey's Manual. Wellesley has these valuable reference books available in electronic form. Link to the electronic edition of | The Prokaryotesthrough Springer ebooks.
Link to the electronic edition of | Bergey's Manualsthrough Springer ebooks.
Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests your perform. It is unlikely we will be able to provide ALL the tests you might wish to perform to identify and evaluate the various roles your soil bacteria may play in its ecosystem, but it is likely that you will be able to identify your organisms to at least the Genera level and discover it least one or two important roles each might play. The DNA sequencing analysis should give you confirmation of the preliminary id you make by traditional means. DNA sequencing should allow you to id your bacteria to the species level. Since your research will also illucidate the role(s) your organisms play in the ecosystem, you will have plenty of evidence to write your final paper.
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab11
Lab 12