BISC209: Lab4: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| (52 intermediate revisions by 3 users not shown) | |||
| Line 4: | Line 4: | ||
==LAB 4: Con't. Project: Soil Microbial Communities & Diversity== | ==LAB 4: Con't. Project: Soil Microbial Communities & Diversity== | ||
Your instructor will return your frozen pcr products containing amplified fragments of 16s | Your instructor will return your frozen pcr products containing amplified fragments of 16s rRNA gene from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rRNA gene fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of ''Escherichia coli'' bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin. | ||
<BR><BR> | <BR><BR> | ||
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the | The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the | ||
| Line 11: | Line 11: | ||
[[Image:toposchemablunt.jpg]] | [[Image:toposchemablunt.jpg]] | ||
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit | We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit will work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form of ''E. coli'' that we will use for separating the amplified 16s rRNA genes from our soil flora.<BR><BR> | ||
[[Image:pcr_Blunt.jpg]] | [[Image:pcr_Blunt.jpg]] | ||
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death) encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. (Cool technology!) | Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death)gene encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. As added insurance that we will select only colonies that are transformed with a plasmid vector with a 16s rRNA gene insert, there is a lacZ gene positioned next to the ccdB gene in the vector. LacZ encodes beta-galactosidase, an enzyme that catalyzes the breakdown of colorless substrates such as Xgal (5-Bromo-4-chloro-3-indolyl beta-Dgalactopyranoside) to a colored cleavage product (in this case, a blue product). Colonies that are transformed with "empty" vectors will not be selected out by plating the colonies on media with kanamycin since the kanamycin resistance gene will be expressed from the empty plasmid vector. However, the promoter for transcription of the ccdB gene AND the lacZ gene is disrupted by the insertion of the 16s DNA insert. Because of this disruption of transcription regulation, the ''lacZ'' gene product (beta-galactosidase) and the ''ccdB'' product (gyrase poison)are not produced in appreciable quantity. This means that cells containing a plasmid vector ''with'' our 16s RNA gene have this disruption of LacZ and ccdB gene regulation and will not be killed by absence of DNA gyrase. They will live and form not-blue colonies because the Xgal in the medium will not be converted to a blue product due to lack of the catalzying enzyme, beta-galactosidase. You will look for white or "not-blue" colonies. (Cool technology!) | ||
==Part A: Using Zero Blunt TOPO PCR Cloning Kit with One Shot TOP 10 Chemically Competent ''E. coli''== | ==Part A: Using Zero Blunt TOPO PCR Cloning Kit with One Shot TOP 10 Chemically Competent ''E. coli''== | ||
PCR cloning requires three steps. <br> | PCR cloning requires three steps. <br> | ||
[[Image: | [[Image:protocloning2.jpg]] | ||
<BR> | <BR><BR> | ||
Procedure:<BR> | We will only clone two pcr products/habitat (one per sampling site). Choose one pcr product per pair to use for cloning and transformation today. Choose the best initial genomic DNA concentration IF that pcr product had good amplification. If the amplification was unsuccessful or weak (judged by staining intensity on the gel), then use the most successful 16s rRNA gene amplification in each habitat sample. <BR><BR> | ||
1. Add | |||
2. Add 1 μL of salt solution<BR> | Procedure: Add the reagents in this order!<BR> | ||
3. Add | 1. Add 2 μl of PCR reaction to a 0.2ml pcr tube (your team color)<BR> | ||
2. Add 1 μL of salt solution ( final conc. 200mM NaCl, 10mM MgCl<sub>2</sub>).<BR> | |||
3. Add 2 μL of purified HPLC water.<BR> | |||
4. Add 1 μL of pCR®II-Blunt-TOPO® cloning vector plasmid. (MAKE sure you pipet this correctly with a P2 and a filter tip!)<BR> | |||
4. Incubate 15 min at room temperature.<BR> | 4. Incubate 15 min at room temperature.<BR> | ||
5. Continue to next step: Transform Oneshot Top10 competent ''E. coli''.<br><BR> | 5. Continue to next step: Transform Oneshot Top10 competent ''E. coli''.<br><BR> | ||
| Line 34: | Line 37: | ||
'''Genotype of OneShot TOP10 Competent Cells:''' ''F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG''<BR><BR> | '''Genotype of OneShot TOP10 Competent Cells:''' ''F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG''<BR><BR> | ||
'''General Handling''': Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting. <BR><BR> | '''General Handling''': Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting(no vortexing). <BR><BR> | ||
| Line 45: | Line 48: | ||
• S.O.C. medium at room temp.(included with the kit)<BR> | • S.O.C. medium at room temp.(included with the kit)<BR> | ||
• 42°C water bath <BR> | • 42°C water bath <BR> | ||
• warm Luria-Bertoni (LB) plates containing 50 μg/ml kanamycin and | • warm Luria-Bertoni (LB) plates containing 50 μg/ml kanamycin and 50μL/ml Xgal (5-Bromo-4-chloro-3-indolyl beta-Dgalactopyranoside) <BR> | ||
• 37°C shaking and non-shaking incubators<BR><BR> | • 37°C shaking and non-shaking incubators<BR><BR> | ||
| Line 53: | Line 56: | ||
• Equilibrate a water bath to 42°C<BR> | • Equilibrate a water bath to 42°C<BR> | ||
• Bring the vial of S.O.C. medium to room temperature.<BR> | • Bring the vial of S.O.C. medium to room temperature.<BR> | ||
• Warm LB plates containing 50 μg/ml kanamycin at 37°C | • Warm LB plates containing 50 μg/ml kanamycin and Xgal at 37°C | ||
for 30 minutes.<BR> | for 30 minutes.<BR> | ||
• Thaw on ice 1 vial of One Shot® cells for each transformation.<BR><BR> | • Thaw on ice 1 vial of One Shot® cells for each transformation.Don't remove them from the -80C until ready for use. <BR><BR> | ||
'''Transformation Procedure'''<BR> | '''Transformation Procedure'''<BR> | ||
| Line 66: | Line 69: | ||
3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).<BR><BR> | 3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).<BR><BR> | ||
4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .<BR><BR> | 4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .<BR><BR> | ||
5. Add 250 μl of room temperature S.O.C. medium.<BR><BR> | 5. Add 250 μl of room temperature S.O.C. medium (it must NOT be cold).<BR><BR> | ||
6. Cap the tube tightly and put the capped tube in a empty non-sterile | 6. Cap the tube tightly and put the capped tube in a empty non-sterile 15 ml. conical tube and shake the tube horizontally (200 rpm) at 37°C for | ||
1 hour. While the shaking is going on, slightly dehydrate 2 LB + kan + Xgal plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then | 1 hour. While the shaking is going on, slightly dehydrate 2 LB + kan + Xgal plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then place the plates in the 37C incubator to prewarm. (The plates must NOT be cold when transformed cells are plated.)<BR><BR> | ||
7. | 7. After the 1 hour incubation of the transformation mix, Use your P200 micropipet to pipet 50 μl from each transformation to the center of a ''prewarmed'' LB + kan+ Xgal plate. Using a disposable sterile plastic spreader, carefully spread the aliquot of cells over the entire surface of the plate.<BR><BR> | ||
8. Repeat step 7 using 200 μL volume of cells. | 8. Repeat step 7 on a new LB + kan + Xgal plate, using a 200 μL volume of transformed cells. You will plate two different volumes to ensure that at least one plate will have well-spaced colonies.<BR><BR> | ||
9. Incubate all plates upside down overnight at 37°C. Remember to label | 9. Incubate all plates upside down overnight at 37°C. Remember to label each plate with all the appropriate information: your initials, lab section, date, your soil sample id and habitat, the type of medium, and the id of the cells and volume used. Refrigerate the remainder of your transformed cells at 4C overnight in case you need to plate a smaller number of cells to achieve isolated colonies. Check your transformations after 12-18 hours (overnight incubation)to be sure of successful transformation. When medium size, ISOLATED colonies, have appeared, refrigerate your transformation plates until LAB 5. DO NOT LEAVE THEM INCUBATING TOO LONG, resulting in overgrown colonies that are not isolated! If you have no transformation or a lawn of growth after the initial overnight incubation, contact your instructor immediately for help. You will need to reisolate by plating a diluted or smaller volume of cells on a new plate or redo the cloning and transformation if neither of the transformations from your habitat is successful. <BR> <BR> | ||
10. An efficient TOPO® Cloning reaction will produce several hundred | 10. An efficient TOPO® Cloning reaction will produce several hundred | ||
colonies. The colonies with inserts will be white or, at least, "not-blue". Look at the map of the cloning vector and the background information description of the cloning and figure out why all colonies should have soil genomic 16s | colonies. The colonies with inserts will be white or, at least, "not-blue". Look at the map of the cloning vector and the background information description of the cloning and figure out why all colonies should have soil genomic 16s rRNA inserts and why those that are not blue are particularly likely to be the ones we want. | ||
==Transformation Media Recipes== | ==Transformation Media Recipes== | ||
'''S.O.C. Medium'''<BR> | '''S.O.C. Medium'''(Super Optimal Broth [SOB] with Catabolite repression (SOC) is SOB with added glucose)<BR> | ||
(may be stored at +4°C or | (may be stored at +4°C or | ||
room temperature)<BR> | room temperature)<BR> | ||
| Line 86: | Line 89: | ||
10 mM MgSO4<BR> | 10 mM MgSO4<BR> | ||
20 mM glucose<BR><BR> | 20 mM glucose<BR><BR> | ||
'''Luria-Bertoni Agar'''<BR> | |||
1% tryptone<BR> | |||
0.5% yeast extract<BR> | |||
1% NaCl <BR> | |||
2% agar for solid medium, none for broth<BR> | |||
50mg/L Kannamycin<BR> | |||
50mg/L Xgal (optional)<BR> | |||
<BR><BR> | |||
=='''Part C: Isolating Culturable Bacteria from soil continued'''== | =='''Part C: Isolating Culturable Bacteria from soil continued'''== | ||
| Line 93: | Line 105: | ||
If you have pure cultures now, continue with the Activities C-1-3 that follow--if not, wait until you do have single colonies in a pure culture.<BR> | If you have pure cultures now, continue with the Activities C-1-3 that follow--if not, wait until you do have single colonies in a pure culture.<BR> | ||
'''Activity C-1: Making Stock Cultures'''<BR><BR> | '''Activity C-1: Making Stock Cultures'''<BR><BR> | ||
Aseptically transfer ~1/8 of a colony from each of your pure cultures to two stock slants. Continue to use the enrichment or general purpose media on which the colony was isolated and grew well. Follow the [[BISC209: Aseptic Transfer | Aseptic Transfer protocol ]]. <BR><BR> | See [[BISC209: Aseptic Transfer | Aseptic Transfer ]] for directions for aseptic transfer of isolated colonies to slants.<BR> | ||
Aseptically transfer ~1/8 of a colony from each of your pure cultures to two stock slants. Continue to use the enrichment or general purpose media on which the colony was isolated and grew well. Follow the [[BISC209: Aseptic Transfer | Aseptic Transfer protocol ]]. Grow these slants at the appropriate temperature until the growth is easily visible and then store them in the test tube rack labeled for your lab section located in the designated area of the cold room.<BR> | |||
Next time you need to make inoculations into multiple culture tubes you will use one of these stock slants. Every time you use a stock slant, immediately replace it by starting another so there is always one untouched one to use and one untouched one in the cold room in case the other becomes contaminated.<BR> | |||
Aseptically transfer another 1/8 of a colony of each pure culture to a new agar plate of the appropriate solid medium. See [[BISC209: Streaking for Isolation | Streaking for Isolation ]]. <BR> <BR> | Aseptically transfer another 1/8 of a colony of each pure culture to a new agar plate of the appropriate solid medium. See [[BISC209: Streaking for Isolation | Streaking for Isolation ]]. <BR> <BR> | ||
Grow these cultures | Grow these plate cultures at the appropriate temperature until you get more mature, healthy, well isolated colonies and then keep the isolation streak plate(s) (sealed with parafilm) in the labeled container in the cold room as a potentially useful backup to your stock slants. <BR><BR> | ||
'''Activity C-2: Gram Stain''' <BR><BR> | |||
'''Activity C-2: Gram Stain and wet mount''' <BR><BR> | |||
Use 1/8 of a well-isolated colony of each bacterial soil isolate to make a smear slide,[[BISC209: Preparing a bacterial smear slide | Smear Slide Preparation]], and to perform a [[BISC209: The Gram Stain | Gram Stain]]. The Gram stain provides information about the cell wall composition, and observing stained cells allows you to assess this bacterial species' shape and characteristic arrangement. You will also be able to determine whether or not all the cells appear identical. If they don't, you may have a pleomorphic organism ''or'' your isolate may not be as pure as you thought. The Gram reaction and the morphologic characteristics that you can assess from this procedure are sometimes crucial in identifying the genera of bacteria that you have isolated. <BR><BR> | Use 1/8 of a well-isolated colony of each bacterial soil isolate to make a smear slide,[[BISC209: Preparing a bacterial smear slide | Smear Slide Preparation]], and to perform a [[BISC209: The Gram Stain | Gram Stain]]. The Gram stain provides information about the cell wall composition, and observing stained cells allows you to assess this bacterial species' shape and characteristic arrangement. You will also be able to determine whether or not all the cells appear identical. If they don't, you may have a pleomorphic organism ''or'' your isolate may not be as pure as you thought. The Gram reaction and the morphologic characteristics that you can assess from this procedure are sometimes crucial in identifying the genera of bacteria that you have isolated. <BR><BR> | ||
Prepare smears by placing 2-3 smears on one slide and stain them simultaneously to save time, as described in [[BISC209: Preparing a bacterial smear slide | Smear Slide Preparation]]. Carefully perform the [[BISC209: The Gram Stain | Gram Stain]] Please follow scrupulously the instructions on proper use of the microscope, particularly when using the oil immersion objective. Those instructions are found at: [[BISC209: Microscopy | Microscopy: Care and Use of the Compound Brightfield Microscope]]<BR> <BR> | Prepare smears by placing 2-3 smears on one slide and stain them simultaneously to save time, as described in [[BISC209: Preparing a bacterial smear slide | Smear Slide Preparation]]. Carefully perform the [[BISC209: The Gram Stain | Gram Stain]] Please follow scrupulously the instructions on proper use of the microscope, particularly when using the oil immersion objective. Those instructions are found at: [[BISC209: Microscopy | Microscopy: Care and Use of the Compound Brightfield Microscope]]<BR> <BR> | ||
If your Gram Stain suggests any of your organisms are still not pure (all genetically identical), talk to your instructor. It is very important that each student has unique pure isolates by LAB 5.<BR> | |||
Consider performing a [[BISC209: wet mounts | ""Wet Mount"" procedure ]] on some of your organisms. A wet mount is useful to observe the shape and arrangement of living organisms as these characteristics can be distorted by heat fixing and staining. It is also useful to determine motility. [[BISC209: wet mounts | Wet Mount procedure ]].<BR><BR> | |||
'''Activity C-3: Performing a Spot Inoculation Technique on Selective Media.'''<BR><BR> | '''Activity C-3: Performing a Spot Inoculation Technique on Selective Media.'''<BR><BR> | ||
| Line 110: | Line 130: | ||
'''Fig: 4C-1.''' Testing of multiple isolates in one plate can be accomplished by dividing a plate into 4 (OR MORE) sections. Be sure the inoculum is placed in the center of each section and that you check the plate for growth regularly. <BR><BR> | '''Fig: 4C-1.''' Testing of multiple isolates in one plate can be accomplished by dividing a plate into 4 (OR MORE) sections. Be sure the inoculum is placed in the center of each section and that you check the plate for growth regularly. <BR><BR> | ||
The inoculation of a few biochemical test media will occur over the next few weeks. You will use the stock cultures that you have started this week or their descendants. Depending on the test, you will need a newly grown agar slant culture, liquid broth culture, or isolation streak plate culture. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. Try to use '''log phase cultures'''. The number of hours it take from inoculation until a bacterial culture moves from log to stationary phase depends on its generation time, the conc. of the inoculum, and other factors. If you have a reasonably fast growing culture, to inoculate your carbohydrate media with a log phase culture, you should make a subculture into an appropriate broth medium about 3-8 hours before you inoculate the test medium. Keep track of how fast each of your soil bacteria grow, on which media,and at what temperature. Since this is an investigative lab | The inoculation of a few biochemical test media will occur over the next few weeks. You will use the stock cultures that you have started this week or their descendants. Depending on the test, you will need a newly grown agar slant culture, liquid broth culture, or isolation streak plate culture. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. Try to use '''log phase cultures'''. The number of hours it take from inoculation until a bacterial culture moves from log to stationary phase depends on its generation time, the conc. of the inoculum, and other factors. If you have a reasonably fast growing culture, to inoculate your carbohydrate media with a log phase culture, you should make a subculture into an appropriate broth medium about 3-8 hours before you inoculate the test medium. Keep track of how fast each of your soil bacteria grow, on which media,and at what temperature. Since this is an investigative lab with no pre-designed outcome, success will require considerable planning and organization as well as copious notetaking. <BR> | ||
| Line 122: | Line 142: | ||
Link to the electronic edition of [http://0-www.springerlink.com.luna.wellesley.edu/content/?k=title%3a%28bergey%27s%29&sortorder=asc&Content+Type=Books | Bergey's Manuals]through Springer ebooks.<BR> | Link to the electronic edition of [http://0-www.springerlink.com.luna.wellesley.edu/content/?k=title%3a%28bergey%27s%29&sortorder=asc&Content+Type=Books | Bergey's Manuals]through Springer ebooks.<BR> | ||
Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests your perform. It is unlikely we will be able to provide ALL the tests you might wish to perform to identify and evaluate the various roles your soil bacteria may play in its ecosystem, but it is likely that you will be able to identify your organisms to at least the Genera level and discover it least one or two important roles each might play. The DNA sequencing analysis should give you confirmation of the preliminary id you make by traditional means. DNA sequencing should allow you to id your bacteria to the species level. Since your research will also illucidate the role(s) your organisms play in the ecosystem, you will have plenty of evidence to write your final paper. | Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests your perform. It is unlikely we will be able to provide ALL the tests you might wish to perform to identify and evaluate the various roles your soil bacteria may play in its ecosystem, but it is likely that you will be able to identify your organisms to at least the Genera level and discover it least one or two important roles each might play. The DNA sequencing analysis should give you confirmation of the preliminary id you make by traditional means. DNA sequencing should allow you to id your bacteria to the species level. Since your research will also illucidate the role(s) your organisms play in the ecosystem, you will have plenty of evidence to write your final paper. | ||
==CLEAN UP== | |||
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.<BR><BR> | |||
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.<BR><BR> | |||
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.<BR><BR> | |||
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.<BR><BR> | |||
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.<BR><BR> | |||
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.<BR><BR> | |||
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.<BR><BR> | |||
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.<BR><BR> | |||
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.<BR><BR> | |||
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.<BR><BR> | |||
11. Take off your lab coat and store it in the blue cabinet with your microscope.<BR><BR> | |||
12. Wash your hands. | |||
==Assignment== | ==Assignment== | ||
'''Write the Introduction to your final paper on Bacterial Diversity in a Soil Community: Roles and relationships '''<BR> | '''Write the Introduction to your final paper on Bacterial Diversity in a Soil Community: Roles and relationships '''<BR> | ||
This graded assignment, due at the beginning of lab next week, is to write the Introduction Section of your final paper. It is often advisable to make the Introduction section of your paper the next-to-the-last section you write (the Abstract should always be written last). However, the advantage of writing the introduction now, before you have most of your results, is that doing so will make you more aware of the "big picture": your ultimate goals in doing all this culture, isolation, characterization and identification of bacteria in a soil community. Writing it now will also help you clarify the potential significance of your findings and | This graded assignment, due at the beginning of lab next week, is to write the Introduction Section of your final paper. It is often advisable to make the Introduction section of your paper the next-to-the-last section you write (the Abstract should always be written last). However, the advantage of writing the introduction now, before you have most of your results, is that doing so will make you more aware of the "big picture": your ultimate goals in doing all this culture, isolation, characterization and identification of bacteria in a soil community. Writing it now will also help you clarify the potential significance of your findings and be able to focus on the more significant tests and results.<BR><BR> | ||
In your introduction you must explain what you are trying to find out and why the answers are important | In your introduction you must explain what you are trying to find out and why the answers are important to an audience that knows nothing about this project and has no reason, ''yet'', to care about soil bacteria. It is the job of the introduction to make non-microbiologists see that identifying and characterizing the bacterial diversity in a soil community is not just an exercise, but that such knowledge might be important in itself and that it might be applied or used in a broader context. For example, what you learn this semester about the impact of particular soil bacteria in their community might allow better understanding of other functional and evolutionary soil community relationships or result in knowledge that might improve the health and functionality of some part of the larger world. Antibiotics, described as the most important discovery leading to improvement of human health in the 20th century, can be discovered and characterized in an investigation such as yours. Antibiotics from bacteria are just one of many, many important possible roles you may discover in the soil bacteria you will study this semester. Your goal in isolating and identifying soil bacterial diversity is to, perhaps, add to the number of roles and relationships we know. | ||
The first step in writing an Introduction to this paper is to be clear on your topic and your goals and to make your audience clear about what they might learn from reading your paper. In lab we stress "doing"--using tool and techniques to address small parts of a much bigger investigative goal. We focus on the small and put the pieces of information together into a bigger picture. When you write the introduction, you must do the opposite. Start with a clear statement of your main topic and overall goal of this investigation and then make your reader care about the answers to the question(s) you address experimentally. Information should move from broad to narrow and from old to new. | The first step in writing an Introduction to this paper is to be clear on your topic and your goals and to make your audience clear about what they might learn from reading your paper. In lab we stress "doing"--using tool and techniques to address small parts of a much bigger investigative goal. We focus on the small and put the pieces of information together into a bigger picture. When you write the introduction, you must do the opposite. Start with a clear statement of your main topic and overall goal of this investigation and then make your reader care about the answers to the question(s) you address experimentally. Information should move from broad to narrow and from old to new. <BR><BR> | ||
Since your reader needs to know a little about what's already known about the types, role and relationship of the bacteria you seek (by culture or by DNA sequencing) from other published studies, you should summarize, quite ''briefly'', early in the introduction, some of the most interesting or seminal findings from previous investigations. Use, primarily, outside published primary sources or review articles. You may not cite the wiki because it's secondary information, as is wikipedia, but both are good places to start. '''This summary of "what's known and how we know it" will require an extensive reference page ''included with this assignment'' created from the many sources you will cite in the introduction'''. We have supplied you with a number of such studies. Pdf files are available in the Reference folder of the First Class conference. You will, certainly, need more than what's available there, but it's a start. <BR><BR> | |||
After you have addressed what you want to know, why it's important and what we already know, then you will BRIEFLY outline the ''general'' strategy you will use to get the knowledge you seek. DO NOT go into detail! Assume your audience has a good science vocabulary, but knows nothing specific about soil bacteria and their possible functional roles. | After you have addressed what you want to know, why it's important and what we already know, then you will BRIEFLY outline the ''general'' strategy you will use to get the knowledge you seek. DO NOT go into detail! Assume your audience has a good science vocabulary, but knows nothing specific about soil bacteria and their possible functional roles. | ||
Refer to appropriate section in the extensive handout, "Guidelines to Scientific Writing" found in the [[BISC209/S10:Resources | Resources]] section of the wiki. Your instructor is available for guidance, and there are the science writing tutors, hired and supervised by the Writing Program. See the Writing Program web page for hours and availability or do to schedule an appointment at [http://www.wellesley.edu/Writing/Program/tutors.html | http://www.wellesley.edu/Writing/Program/tutors.html].<BR><BR> | Refer to appropriate section in the extensive handout, "Guidelines to Scientific Writing" found in the [[BISC209/S10:Resources | Resources]] section of the wiki. Your instructor is available for guidance, and there are the science writing tutors, hired and supervised by the Writing Program, also available for help. See the Writing Program web page for hours and availability or do to schedule an appointment at [http://www.wellesley.edu/Writing/Program/tutors.html | http://www.wellesley.edu/Writing/Program/tutors.html].<BR><BR> | ||
'''Continue monitoring and following the appropriate protocols to | '''Continue monitoring and following the appropriate protocols to enrich and isolate the culturable bacteria.''' Beginning in lab 5 and continuing through the remaining semester, you will be testing your isolates for their potential role(s) in the soil community using a variety of protocols. The antibiotic producer protocol requires starting cultures on agar media 5-7 days prior to lab 5 and broth cultures in exponential growth phase ready to use in lab 5. Read the protocol carefully and start your cultures at the appropriate time. You find the various characterization tests in the protocol section of this wiki: <BR> | ||
[[BISC209: Stains | Stains (Special): Endospore, Acid fast, Capsule, and Flagella]]<BR> | |||
[[BISC209: Motility | Motility Tests]]<BR> | |||
[[BISC209: Enzyme tests |Enzyme tests ]]<BR> | |||
[[BISC209: Roles of soil Microbes |Tests to determine the role of a soil isolate]]<BR> | |||
[[BISC209: wet mounts | Wet Mount procedure ]].<BR><BR> | |||
A few minutes prior to Lab 5 someone from each sampling site should go to the greenhouse with gloves and a plastic sandwich bag (available in the lab) and collect 2 leaves from an inconspicuous location on one or two plants around your sampling site. We will be using these leaves to make leaf disks for the cellulose degradation protocol found in [[BISC209: Roles of soil Microbes |Tests to determine the role of a soil isolate]]; therefore, it is important that you do not contaminate the surface of your leaf with skin flora. If you don't have time to do this before lab, you can send someone in your group down to collect leaves during lab.<BR> | |||
==Links to Labs== | ==Links to Labs== | ||
Latest revision as of 10:26, 17 June 2010
LAB 4: Con't. Project: Soil Microbial Communities & Diversity
Your instructor will return your frozen pcr products containing amplified fragments of 16s rRNA gene from many of the species of soil bacteria in your soil sample. Today you will insert your bacterial 16s rRNA gene fragments into a patented cloning vector (pCR-BluntII TOPO®) and then transform that vector into a special genetically engineered strain of Escherichia coli bacteria that will express a vector gene for kanamycin resistance, allowing us to select for transformants on media containing kanamycin.
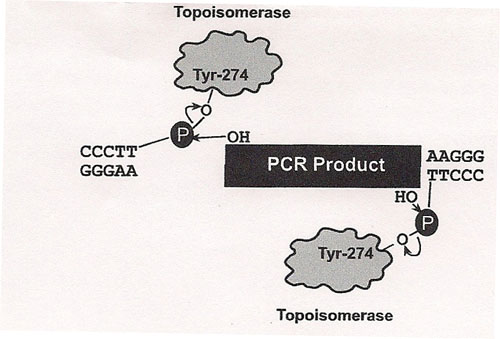
The principle behind TOPO® cloning is the enzyme DNA topoisomerase I, which will function in this system both as a restriction enzyme and as a ligase. Its biological role is to cleave and rejoin DNA during replication. Vaccinia virus topoisomerase I specifically recognizes the pentameric sequence 5´-(C/T)CCTT-3´ and forms a covalent bond with the
phosphate group attached to the 3´ thymidine. It cleaves one DNA strand, enabling the DNA to unwind. The enzyme then religates the ends of the cleaved strand and releases itself from the DNA. To harness the religating activity of topoisomerase, TOPO® vectors are provided linearized with topoisomerase I covalently bound to each 3´ phosphate. This enables the vectors to quickly ligate DNA sequences with compatible ends.
We used a polymerase that creates blunt ended DNA fragments rather than using TaQ. Taq polymerase makes fragments with 3' T overhangs; therefore, complementary single stranded A rich "sticky ends" allow ligation. Blunt ends require a different Blunt-fragment cloning protocol. Invitrogen's Zero Blunt® TOPO® PCR Cloning Kit will work well for us. It has several (T7, SP6, and M13 forward and reverse) priming sites for directing sequencing to the appropriate region and it has two resistance genes, Kanamycin and Zeocin, for selecting clones in a genetically engineered form of E. coli that we will use for separating the amplified 16s rRNA genes from our soil flora.
Additionally, the cloning system we will use contains a background reducer, a lethal ccdB (control of cell death)gene encoding a ccdB protein that poisons bacterial DNA gyrase, causing degradation of the host chromosome and cell death. However, when one of our pcr products is ligated into the vector, the ccdB gene is disrupted, enabling these recombinant colonies to grow while other non-transformants do not. As added insurance that we will select only colonies that are transformed with a plasmid vector with a 16s rRNA gene insert, there is a lacZ gene positioned next to the ccdB gene in the vector. LacZ encodes beta-galactosidase, an enzyme that catalyzes the breakdown of colorless substrates such as Xgal (5-Bromo-4-chloro-3-indolyl beta-Dgalactopyranoside) to a colored cleavage product (in this case, a blue product). Colonies that are transformed with "empty" vectors will not be selected out by plating the colonies on media with kanamycin since the kanamycin resistance gene will be expressed from the empty plasmid vector. However, the promoter for transcription of the ccdB gene AND the lacZ gene is disrupted by the insertion of the 16s DNA insert. Because of this disruption of transcription regulation, the lacZ gene product (beta-galactosidase) and the ccdB product (gyrase poison)are not produced in appreciable quantity. This means that cells containing a plasmid vector with our 16s RNA gene have this disruption of LacZ and ccdB gene regulation and will not be killed by absence of DNA gyrase. They will live and form not-blue colonies because the Xgal in the medium will not be converted to a blue product due to lack of the catalzying enzyme, beta-galactosidase. You will look for white or "not-blue" colonies. (Cool technology!)
Part A: Using Zero Blunt TOPO PCR Cloning Kit with One Shot TOP 10 Chemically Competent E. coli
PCR cloning requires three steps.
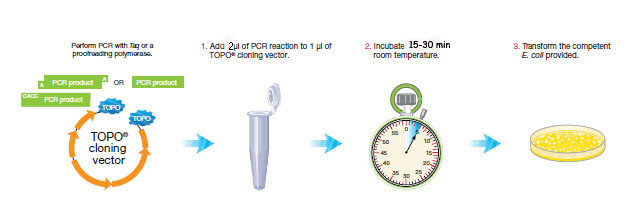
We will only clone two pcr products/habitat (one per sampling site). Choose one pcr product per pair to use for cloning and transformation today. Choose the best initial genomic DNA concentration IF that pcr product had good amplification. If the amplification was unsuccessful or weak (judged by staining intensity on the gel), then use the most successful 16s rRNA gene amplification in each habitat sample.
Procedure: Add the reagents in this order!
1. Add 2 μl of PCR reaction to a 0.2ml pcr tube (your team color)
2. Add 1 μL of salt solution ( final conc. 200mM NaCl, 10mM MgCl2).
3. Add 2 μL of purified HPLC water.
4. Add 1 μL of pCR®II-Blunt-TOPO® cloning vector plasmid. (MAKE sure you pipet this correctly with a P2 and a filter tip!)
4. Incubate 15 min at room temperature.
5. Continue to next step: Transform Oneshot Top10 competent E. coli.
Part B Transforming TOPO Competent E. coli
Genotype of OneShot TOP10 Competent Cells: F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG
General Handling: Be extremely gentle when working with competent cells. Competent cells have been chemically treated to make their cell walls and membranes more porous so they are fragile and highly sensitive to changes in temperature. They can be easily lysed by too vigorous pipetting. Transformation should be started immediately following the thawing of the cells on ice. Mix by swirling or tapping the tube gently, not by pipetting(no vortexing).
Transforming One Shot® Competent Cells
Introduction: Once you have performed the TOPO® Cloning reaction, you will transform your pCR®-Blunt II-TOPO® construct into TOPO10 competent E. coli provided with your kit.
You will need to gather:
In addition to general microbiological supplies (e.g. petri dish with ethanol, glass spreader or sterile glass beads), you will need the following reagents and equipment.
• TOPO® Cloning reaction from Performing the TOPO® Cloning Reaction
• S.O.C. medium at room temp.(included with the kit)
• 42°C water bath
• warm Luria-Bertoni (LB) plates containing 50 μg/ml kanamycin and 50μL/ml Xgal (5-Bromo-4-chloro-3-indolyl beta-Dgalactopyranoside)
• 37°C shaking and non-shaking incubators
Preparing for Transformation
For each transformation, you will need one vial of competent cells and two
selective plates.
• Equilibrate a water bath to 42°C
• Bring the vial of S.O.C. medium to room temperature.
• Warm LB plates containing 50 μg/ml kanamycin and Xgal at 37°C
for 30 minutes.
• Thaw on ice 1 vial of One Shot® cells for each transformation.Don't remove them from the -80C until ready for use.
Transformation Procedure
1. Add 2 μl of the TOPO® Cloning reaction when it is completed into a vial of One Shot® Chemically Competent E. coli and mix gently by swirling. Do not mix by pipetting up and down!
2. Incubate on ice for 10 minutes.
Note: Longer incubations on ice do not seem to have any affect on transformation
efficiency. The length of the incubation is at the user’s discretion.
3. Heat-shock the cells for 30 seconds exactly at 42°C in the heatblock (without shaking).
4. Immediately (take your ice bucket with you to the heat block) transfer the tubes to ice .
5. Add 250 μl of room temperature S.O.C. medium (it must NOT be cold).
6. Cap the tube tightly and put the capped tube in a empty non-sterile 15 ml. conical tube and shake the tube horizontally (200 rpm) at 37°C for
1 hour. While the shaking is going on, slightly dehydrate 2 LB + kan + Xgal plates by placing them with lids slightly agar in the hood with the blower on for 10 min. Then place the plates in the 37C incubator to prewarm. (The plates must NOT be cold when transformed cells are plated.)
7. After the 1 hour incubation of the transformation mix, Use your P200 micropipet to pipet 50 μl from each transformation to the center of a prewarmed LB + kan+ Xgal plate. Using a disposable sterile plastic spreader, carefully spread the aliquot of cells over the entire surface of the plate.
8. Repeat step 7 on a new LB + kan + Xgal plate, using a 200 μL volume of transformed cells. You will plate two different volumes to ensure that at least one plate will have well-spaced colonies.
9. Incubate all plates upside down overnight at 37°C. Remember to label each plate with all the appropriate information: your initials, lab section, date, your soil sample id and habitat, the type of medium, and the id of the cells and volume used. Refrigerate the remainder of your transformed cells at 4C overnight in case you need to plate a smaller number of cells to achieve isolated colonies. Check your transformations after 12-18 hours (overnight incubation)to be sure of successful transformation. When medium size, ISOLATED colonies, have appeared, refrigerate your transformation plates until LAB 5. DO NOT LEAVE THEM INCUBATING TOO LONG, resulting in overgrown colonies that are not isolated! If you have no transformation or a lawn of growth after the initial overnight incubation, contact your instructor immediately for help. You will need to reisolate by plating a diluted or smaller volume of cells on a new plate or redo the cloning and transformation if neither of the transformations from your habitat is successful.
10. An efficient TOPO® Cloning reaction will produce several hundred
colonies. The colonies with inserts will be white or, at least, "not-blue". Look at the map of the cloning vector and the background information description of the cloning and figure out why all colonies should have soil genomic 16s rRNA inserts and why those that are not blue are particularly likely to be the ones we want.
Transformation Media Recipes
S.O.C. Medium(Super Optimal Broth [SOB] with Catabolite repression (SOC) is SOB with added glucose)
(may be stored at +4°C or
room temperature)
2% Tryptone
0.5% Yeast Extract
10 mM NaCl
2.5 mM KCl
10 mM MgCl2
10 mM MgSO4
20 mM glucose
Luria-Bertoni Agar
1% tryptone
0.5% yeast extract
1% NaCl
2% agar for solid medium, none for broth
50mg/L Kannamycin
50mg/L Xgal (optional)
Part C: Isolating Culturable Bacteria from soil continued
Obtaining Pure Cultures of Isolates
By this week we hope that you have several well isolated, pure colonies from many of your isolation streak plates. If so, congratulations!. Make sure that these potentially pure cultures have colonies that look like the original source colony and that all of the colonies look the same (they can be of different size). If you are having trouble obtaining well isolated colonies, consult with your instructor. You may need to return to the original plates, to try isolation streaking again.
Your goal is that each person in a team of 4 students sampling from the same habitat will isolate and identify examples of soil bacteria from as many DIFFERENT types of enrichment media as possible. Coordinate with your team members as you attempt to isolate colonies that look different from each other and that include at least one or two potential antibiotic producers (Actinomycetes, Myxobacteria, Bacillus: aerobic spore formers) as well as the other different interesting types of bacteria. It would be great if each team identified 16 different bacteria in your soil habitat, including at least one from each of the groups that we have enrichment media to find. That may not be possible, but it will make your poster presentation at the end of this project more interesting if everyone has some unique bacteria.
If you have pure cultures now, continue with the Activities C-1-3 that follow--if not, wait until you do have single colonies in a pure culture.
Activity C-1: Making Stock Cultures
See Aseptic Transfer for directions for aseptic transfer of isolated colonies to slants.
Aseptically transfer ~1/8 of a colony from each of your pure cultures to two stock slants. Continue to use the enrichment or general purpose media on which the colony was isolated and grew well. Follow the Aseptic Transfer protocol . Grow these slants at the appropriate temperature until the growth is easily visible and then store them in the test tube rack labeled for your lab section located in the designated area of the cold room.
Next time you need to make inoculations into multiple culture tubes you will use one of these stock slants. Every time you use a stock slant, immediately replace it by starting another so there is always one untouched one to use and one untouched one in the cold room in case the other becomes contaminated.
Aseptically transfer another 1/8 of a colony of each pure culture to a new agar plate of the appropriate solid medium. See Streaking for Isolation .
Grow these plate cultures at the appropriate temperature until you get more mature, healthy, well isolated colonies and then keep the isolation streak plate(s) (sealed with parafilm) in the labeled container in the cold room as a potentially useful backup to your stock slants.
Activity C-2: Gram Stain and wet mount
Use 1/8 of a well-isolated colony of each bacterial soil isolate to make a smear slide, Smear Slide Preparation, and to perform a Gram Stain. The Gram stain provides information about the cell wall composition, and observing stained cells allows you to assess this bacterial species' shape and characteristic arrangement. You will also be able to determine whether or not all the cells appear identical. If they don't, you may have a pleomorphic organism or your isolate may not be as pure as you thought. The Gram reaction and the morphologic characteristics that you can assess from this procedure are sometimes crucial in identifying the genera of bacteria that you have isolated.
Prepare smears by placing 2-3 smears on one slide and stain them simultaneously to save time, as described in Smear Slide Preparation. Carefully perform the Gram Stain Please follow scrupulously the instructions on proper use of the microscope, particularly when using the oil immersion objective. Those instructions are found at: Microscopy: Care and Use of the Compound Brightfield Microscope
If your Gram Stain suggests any of your organisms are still not pure (all genetically identical), talk to your instructor. It is very important that each student has unique pure isolates by LAB 5.
Consider performing a ""Wet Mount"" procedure on some of your organisms. A wet mount is useful to observe the shape and arrangement of living organisms as these characteristics can be distorted by heat fixing and staining. It is also useful to determine motility. Wet Mount procedure .
Activity C-3: Performing a Spot Inoculation Technique on Selective Media.
Since you only have about 1/8 to 1/4 of the original colonies used in the activities above for each organism left, be judicious when you "confirm" the Gram reaction and check for contaminants by spot inoculation on solid selective and differential: EMB and PEA media. Consult Use of Selective, Differential, Enrichment Media. You can test all your isolates, but if you do not see growth on either EMB or PEA you should be able to explain this outcome). Use a marker to divide the bottom of each plate into 4-8 sections and organize a labeling system in your lab notebook and on the plate so you can easily identify where you placed each of your soil isolates. You will spot inoculate the middle of each quadrant by taking a tiny amount of growth from the source isolate and inoculating with a single thin zig-zag line in the center of a section. See the illustration below of a plate testing 4 samples. If you do not have enough of the colony left to do this today, label the plates, and place them in your section of the cold room for use next lab when your stock subcultures have restored your supply of bacteria to test. Remember that bacteria reproduce asexually by binary fission so that if a colony comes from a single cell and you only use one colony or its descendants for all of your tests, you have used a genetically identical population (excluding spontaneous mutations) of cells for all of your tests over the semester.


Fig: 4C-1. Testing of multiple isolates in one plate can be accomplished by dividing a plate into 4 (OR MORE) sections. Be sure the inoculum is placed in the center of each section and that you check the plate for growth regularly.
The inoculation of a few biochemical test media will occur over the next few weeks. You will use the stock cultures that you have started this week or their descendants. Depending on the test, you will need a newly grown agar slant culture, liquid broth culture, or isolation streak plate culture. You will need to plan ahead to prepare the appropriate cultures so that they will be ready to use when needed. Try to use log phase cultures. The number of hours it take from inoculation until a bacterial culture moves from log to stationary phase depends on its generation time, the conc. of the inoculum, and other factors. If you have a reasonably fast growing culture, to inoculate your carbohydrate media with a log phase culture, you should make a subculture into an appropriate broth medium about 3-8 hours before you inoculate the test medium. Keep track of how fast each of your soil bacteria grow, on which media,and at what temperature. Since this is an investigative lab with no pre-designed outcome, success will require considerable planning and organization as well as copious notetaking.
It is essential that your stock slant cultures remain fresh and uncontaminated. Don't forget to replace a streak plate or slant frequently, using the media on which you successfully isolated that type of bacterium.
Remember: Each time you use a stock slant to inoculate more media, you should first make a fresh stock slant (this prevents accidental contamination as you perform the remaining inoculations as well as providing a replacement for the used stock). Grow the new stock for the appropriate amount of time, and then store it in the cold room until needed.
Classification of Unknown Isolates
Your most important resource for identification will be the reference manuals: THE PROKARYOTES and Bergey's Manual. Wellesley College has these valuable reference books available in electronic form. Link to the electronic edition of | The Prokaryotesthrough Springer ebooks.
Link to the electronic edition of | Bergey's Manualsthrough Springer ebooks.
Use these reference resources to decipher the meaning of the results of the morphologic, metabolic, physical and other tests your perform. It is unlikely we will be able to provide ALL the tests you might wish to perform to identify and evaluate the various roles your soil bacteria may play in its ecosystem, but it is likely that you will be able to identify your organisms to at least the Genera level and discover it least one or two important roles each might play. The DNA sequencing analysis should give you confirmation of the preliminary id you make by traditional means. DNA sequencing should allow you to id your bacteria to the species level. Since your research will also illucidate the role(s) your organisms play in the ecosystem, you will have plenty of evidence to write your final paper.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
Write the Introduction to your final paper on Bacterial Diversity in a Soil Community: Roles and relationships
This graded assignment, due at the beginning of lab next week, is to write the Introduction Section of your final paper. It is often advisable to make the Introduction section of your paper the next-to-the-last section you write (the Abstract should always be written last). However, the advantage of writing the introduction now, before you have most of your results, is that doing so will make you more aware of the "big picture": your ultimate goals in doing all this culture, isolation, characterization and identification of bacteria in a soil community. Writing it now will also help you clarify the potential significance of your findings and be able to focus on the more significant tests and results.
In your introduction you must explain what you are trying to find out and why the answers are important to an audience that knows nothing about this project and has no reason, yet, to care about soil bacteria. It is the job of the introduction to make non-microbiologists see that identifying and characterizing the bacterial diversity in a soil community is not just an exercise, but that such knowledge might be important in itself and that it might be applied or used in a broader context. For example, what you learn this semester about the impact of particular soil bacteria in their community might allow better understanding of other functional and evolutionary soil community relationships or result in knowledge that might improve the health and functionality of some part of the larger world. Antibiotics, described as the most important discovery leading to improvement of human health in the 20th century, can be discovered and characterized in an investigation such as yours. Antibiotics from bacteria are just one of many, many important possible roles you may discover in the soil bacteria you will study this semester. Your goal in isolating and identifying soil bacterial diversity is to, perhaps, add to the number of roles and relationships we know.
The first step in writing an Introduction to this paper is to be clear on your topic and your goals and to make your audience clear about what they might learn from reading your paper. In lab we stress "doing"--using tool and techniques to address small parts of a much bigger investigative goal. We focus on the small and put the pieces of information together into a bigger picture. When you write the introduction, you must do the opposite. Start with a clear statement of your main topic and overall goal of this investigation and then make your reader care about the answers to the question(s) you address experimentally. Information should move from broad to narrow and from old to new.
Since your reader needs to know a little about what's already known about the types, role and relationship of the bacteria you seek (by culture or by DNA sequencing) from other published studies, you should summarize, quite briefly, early in the introduction, some of the most interesting or seminal findings from previous investigations. Use, primarily, outside published primary sources or review articles. You may not cite the wiki because it's secondary information, as is wikipedia, but both are good places to start. This summary of "what's known and how we know it" will require an extensive reference page included with this assignment created from the many sources you will cite in the introduction. We have supplied you with a number of such studies. Pdf files are available in the Reference folder of the First Class conference. You will, certainly, need more than what's available there, but it's a start.
After you have addressed what you want to know, why it's important and what we already know, then you will BRIEFLY outline the general strategy you will use to get the knowledge you seek. DO NOT go into detail! Assume your audience has a good science vocabulary, but knows nothing specific about soil bacteria and their possible functional roles.
Refer to appropriate section in the extensive handout, "Guidelines to Scientific Writing" found in the Resources section of the wiki. Your instructor is available for guidance, and there are the science writing tutors, hired and supervised by the Writing Program, also available for help. See the Writing Program web page for hours and availability or do to schedule an appointment at | http://www.wellesley.edu/Writing/Program/tutors.html.
Continue monitoring and following the appropriate protocols to enrich and isolate the culturable bacteria. Beginning in lab 5 and continuing through the remaining semester, you will be testing your isolates for their potential role(s) in the soil community using a variety of protocols. The antibiotic producer protocol requires starting cultures on agar media 5-7 days prior to lab 5 and broth cultures in exponential growth phase ready to use in lab 5. Read the protocol carefully and start your cultures at the appropriate time. You find the various characterization tests in the protocol section of this wiki:
Stains (Special): Endospore, Acid fast, Capsule, and Flagella
Motility Tests
Enzyme tests
Tests to determine the role of a soil isolate
Wet Mount procedure .
A few minutes prior to Lab 5 someone from each sampling site should go to the greenhouse with gloves and a plastic sandwich bag (available in the lab) and collect 2 leaves from an inconspicuous location on one or two plants around your sampling site. We will be using these leaves to make leaf disks for the cellulose degradation protocol found in Tests to determine the role of a soil isolate; therefore, it is important that you do not contaminate the surface of your leaf with skin flora. If you don't have time to do this before lab, you can send someone in your group down to collect leaves during lab.
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab11
Lab 12