BISC220/S10: Mod 1 Lab 1
Background Information
The purification of an enzyme is often required in order to study its physical characteristics and the reaction it catalyzes. Purification is essentially a concentrating process in which the concentration of the target enzyme is increased while other proteins are eliminated. The enrichment process also eliminates metabolites and other cellular constituents that may influence the enzyme structure and/or the reaction. Background information on protein purification can be found below and also in your textbook.
During this sequence of laboratory exercises, we will be partially purifying and characterizing the enzyme β-galactosidase from Escherichia coli, strain BL21(pET-14b). Although we will not be concerned with regulation of gene expression, it is of interest that experimentation on the production of β-galactosidase by E. coli ultimately led Francois Jacob and Jacques Monod to propose the 'operon' model for the regulation of gene expression. If you are interested in learning more about transcription regulation and early work on the genetic analysis of the lac-operon in E. coli, your text is a good additional source.
For this first series of lab exercises, we have chosen to study the enzyme β-galactosidase for a variety of reasons: it is a convenient enzyme for learning some of the basic techniques in enzymology because the enzyme is easily available in large quantities from microbial cells; the gene has been cloned; the crystal structure has been solved; it is relatively stable; the specific activity assay generally used is reliable and convenient (Jacobson et. al., 1994; Ring, 1990; Wallenfels and Weil, 1972).
Βeta-galactosidase allows E. coli to grow in a culture medium containing the sugar lactose as the sole source of energy. The enzyme hydrolyzes lactose into glucose and galactose:
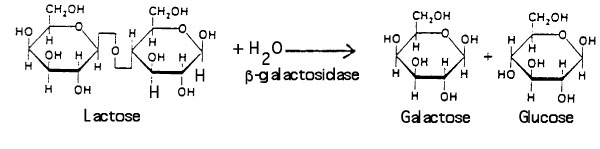
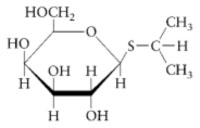
The purification of β-galactosidase is facilitated when E. coli produce large quantities of the enzyme. Growth on lactose as a carbon source is not a sufficient means of insuring abundant production of the enzyme since the expression of β-galactosidase is controlled. β-galactosidase production remains partially repressed even when cells are grown in the presence of the substrate lactose. It is possible, however, to transform genetically engineered strains of E. coli with a plasmid that expresses high levels of β-galactosidase. The plasmid fuses a strong promoter upstream of the β-galactosidase gene, lacZ. The gene promoter (and the gene’s over expression) is regulated by isopropyl-β-D-thiogalactoside (IPTG), the structure of which is shown below.
In addition to allowing the production of large quantities of β-galactosidase, the lacZ gene on the plasmid, is modified so that the primary sequence of the enzyme is extended on the amino terminus with six histidines. This 6xHis tail can be exploited to purify the protein using metal chelate affinity chromatography (Kagedal, 1998). See Lab 2 for additional background information on metal chelate affinity chromatography.
Our goals for Lab 1 are:
- Calibrate our micropipets,
- Induce E. coli BL21(pET-14b) cells to produce large quantities of 6xHis tagged β-galactosidase, and
- Learn how to model macromolecules and analyze their sequences, using the computer programs RasMol and ClustalW.
Tutorials
RasMol Tutorial
ClustalW Tutorial
References:
Jacobson RH, Zhang XJ, DuBose RF, Matthews BW (1994) Three-dimensional structure of β-galactosidase from E. coli. Nature. 369:761-6.
Karp G (2005) Cell and Molecular Biology Concepts and Experiments, 4th edition, John Wiley & Sons, Inc.,Hoboken.
Ring M, Huber RE (1990) Multiple replacements establish the importance of tyrosine-503 in beta-galactosidase (Escherichia coli). Arch Biochem Biophys. 283:342-50.
Wallenfels K, Weil R (1972) “β-galactosidase” In The Enzymes (Boyer PD, ed), Vol VII, 3rd ed, Academic Press, New York, pp 617-663.
Protocols
THE INDUCTION OF β-GALACTOSIDASE
You will be provided with approximately 200 ml of E. coli BL21 (pET-14b) in log phase growth in Luria broth (LB) media. You will induce the cells of this genetically engineered strain to increase production of 6xHis-β-galactosidase by adding IPTG to the cells and incubating them at 37°C for one hour. You will collect the cells by centrifugation and freeze the cell pellet until the next lab period.
