BISC220/S10: Mod 3 Lab 9
Cell Culture Techniques
Tissue culture (cell culture) was introduced at the beginning of the 20th century as a method for studying animal cells. The term tissue culture is derived from the original technique that used fragments of tissue surrounded by a nutrient medium. At first cell cultures were mainly the result of migration of cells from a tissue fragment into the surrounding medium, but by the 1950's primary cell cultures could be created from dispersed cells in liquid nutrient medium. Passaging of a primary cell cultures creates a cell line. Cell lines made from normal cells cannot be maintained forever, but immortalized cell lines derived from cancer cells or otherwise abnormal can often be passaged indefinitely.
Cell culture is an important technique in the study of cellular physiology. Cell culture facilitates the investigation of cell structure, reproduction, protein synthesis, energy metabolism, cell-cell interactions and environmental influences on growth. One of the advantages of using cell culture is that the physiochemical environment (pH, temperature, osmotic pressure, O2 and CO2 tension) can be precisely controlled. Growing cells in culture also enables a specific type of cell to be exposed directly to a test agent without interference from excretion or metabolic breakdown in a living animal.
A disadvantage associated with cell culture is that all manipulations must be performed under strict aseptic conditions to prevent contamination. A contaminated culture is useless. The validity of the cultured cell as an accurate model of in vivo physiologic function may also be questioned, since the 3-dimensional geometry of the cells in the original tissue is not strictly maintained and some of the cell lines are of metabolically abnormal cancer cells.
Cells in cell culture are suspended in a nutrient liquid medium containing amino acids, vitamins, salts, glucose and blood serum to support the growth of the cells. Serum is a necessary additive to cell culture media because it supplies the cells with many growth factors that they need to proliferate and survive in vitro. The culture is then incubated at 37°C in an environment of 95% air and 5% CO2. Under these conditions, some of the tissue cells will create a monolayer that adheres to the culture flask and forms the basis of the primary culture.
Many cultured cells adhere to a substrate surface in the culture flask. The most common substrate materials are glass, or plastic that has been treated with chemicals or radiation to make the surface more hydrophilic so the cells will readily adhere. When cells occupy all of the substrate surface area, a state of confluence is reached. Once a confluent monolayer of cells has formed in the culture vessel, normal cells stop dividing. At this time the cells need to be supplied with fresh medium or sub-cultured to reduce the density of cells in the culture flask so growth can resume. After the first sub-culture or passage, the primary culture becomes a cell line. With each subculture, faster growing cells will soon predominate as the slower growing cells are diluted out of the population. This results in a relatively uniform population of cells available for experimentation. The number of times a cell line can be successfully subcultured depends on the type of cell. Many normal cells may only be subcultured a limited number of times, while most cancer cells can be sub-cultured indefinitely.
In the lab today, each group will be given a culture of 3T3 fibroblast cells derived from mouse embryos. Each group member will aseptically create a subculture. The cells will be supplied at or near confluence, so the rate of growth of the cells has ceased and subculture is necessary. Sub-culture of the fibroblasts involves removal of the medium from the flask, exposure of the cells to the protease, trypsin, to disassociate them from the substrate surface, and dispersal of the cells at the proper density in fresh medium. You should be able to prepare 2-3 cell culture flasks seeded at 2.4 x 104 cells/ml from the original culture.
Materials Provided for Cell Culture:
- Dulbecco's modified Eagle Medium containing salts, amino acids, vitamins and glucose, with 10% calf serum (sterile)
- Phosphate buffered saline, pH=7.4 (sterile) 137mM NaCl, 2.7 mM KCl, 10mM Na2HPO4, 2 mM KH2PO4
- 0.25% Trypsin in sterile PBS
- Sterile pipettes: 1 ml, 5 ml and 10 ml
- 0.1% Trypan Blue stain
- Hemocytometer & coverslip
- Microscope
- Tissue culture flasks, 25 cm2
- Culture of 3T3 fibroblasts
Protocol
All cell culture manipulations should be performed in one of the laminar flow hoods located in rooms 302 and 304. Your instructor will demonstrate its proper use. Proper aseptic technique will reduce the chance of contamination of your cell culture.
- Aspirate the medium from the tissue culture flask using a sterile Pasteur pipet. Discard the medium in a waste container and the pipet in an autoclave bag.
- Using a sterile 5.0 ml pipette, add 5.0 ml of sterile phosphate buffered saline (PBS) to the side of the flask opposite the cells. Tilt the flask to rinse the cells with the saline. Aspirate the PBS and discard it into the waste.
- Pour 3.0 ml of the trypsin solution to the side of the flask opposite the cells. Place the flask on its side so the cells are covered completely. Gently tilt the flask for 30-45 seconds only. Aspirate the trypsin and discard it to the waste.
- Incubate the flask at 37°C in the incubator with 5% CO2 for approximately 15 minutes. Observe the cells under the microscope to make sure that they have “rounded up”.
- Using a sterile 5.0 ml pipette, add 5.0 ml of medium to the cell side of the flask and disperse the cells by repeatedly pipeting over the monolayer surface. Then pipette the cell suspension up and down several times with the pipette tip resting on the bottom corner of the flask. Be careful not to introduce air bubbles. These manipulations will create a cell suspension suitable for counting.
- To determine how many cells/ml are in this stock culture you must remove a small aliquot for counting. Mix well and then aseptically remove a few drops of your cell culture using a sterile Pasteur pipet. Place three drops (with the Pasteur pipet held vertically) of the cell culture into a microfuge tube. Discard the pipet into the waste container. Do not replace any excess culture back into the culture flask. Leave your labeled culture flask in the hood with the top on and take the microfuge tube back to your lab bench. Add 1 drop of trypan blue stain to the 3 drops of your cell culture in the microfuge tube to make a 3:4 cell dilution. Trypan blue will stain dead cells blue so that they can be distinguished from living cells microscopically. Mix well.
- Place a special hemocytometer cover slip on the hemocytometer chamber. NOTE: These coverslips are NOT disposable! Load the mixed cell suspension-trypan blue solution into the chamber being very careful to completely fill, but not overfill, the chamber. Ask your instructor for help if you are unsure about how to do this.
- Using the 10X objective (total magnification 100x), count all the living (non-blue) cells in each of the 4 large squares numbered 1-4 in Figure 1. Determine the average number of cells per square. This is the number of cells in 10-4 ml. Therefore:
Note: The dilution factor is the reciprocal of the dilution
Figure 1 shows the microscopic grid engraved on the Hemocytometer counting chamber. The entire ruled area is 9 mm2 with a depth of 0.1 mm when properly loaded. The volume of 1 large square (eg. #1) is 10-4 ml. (From Bauer, J.D. , et al. Bray's Clinical Lab Methods)
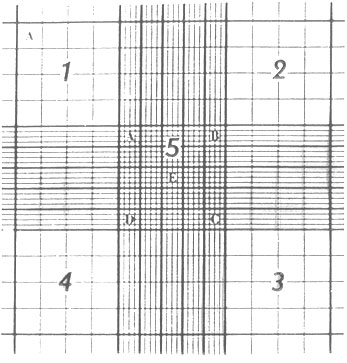
Figure 1. Hemocytometer Cell Counting Chamber
9. Calculate the appropriate dilution of the cell suspension to yield 5.0 ml of culture at a cell density of 2.4 x 104 cells/ml. You should use the equation: V1 x C1 = V2 x C2
For example: If you have 8 x 104 cells/ ml in your original culture, and you want to make 5.0 ml of a new culture at 2.4 x 104 cells/ml, you must solve the above equation for V1.
V1 = 5.0 ml x (2.4 x 104 cells/ml)/ 8 x 104 cells/ml
Place 3.5 ml of fresh media into a 25 cm2 tissue culture flask. Mix the original culture using a sterile 5.0 ml pipette and add 1.5 ml of culture to the 3.5 ml of media to prepare a 5.0 ml cell culture seeded at 2.5 x 104 cells/ml.
10. Each person in the group should add the calculated amount of new media to the calculated amount of the counted cell culture to create a new culture with 2.4 x 104 cells/ml in a new 25 cm2 tissue culture flask with a 5ml volume of new passaged cells. Cap the flask but do not tighten the cap all the way to allow for the entry of air and CO2 during incubation. Label the flask on the side with your name, date, cell type and lab section. Place your cell culture back in the 37°C CO2 incubator on the shelf designated for your lab section.
11. After 3 or 4 days, it will be necessary to "feed" your culture by removing the old media and adding fresh media. Extra media will be stored in the refrigerator in room 304, and an area will be set up in one of the laminar flow hoods in room 304. Please work in the laminar flow hood to avoid contamination. Using a sterile pipette, remove the media from the flask and discard to waste. Using a new sterile pipette, add 5.0 ml of fresh media to the flask and return the culture to the incubator in Room 308.
For lab next week:
Next week you will be treating cultured fibroblasts with different concentrations of cytochalasin B and observing its effects on the process of pinocytosis and on the cytoskeleton of your 3T3 fibroblasts. Your instructor will assign your group’s cytocholasin B concentrations before you leave today. You will need to make dilutions of a stock solution of cytochalasin B to obtain your assigned test concentrations. Please look over the background information and the protocol for next week’s lab and think about your drug dilution strategy before coming to lab next week. Although there is nothing to turn in for next week, please do enough background research to form a hypothesis about how treatment with cytochalasin B might affect your cells, particularly specific aspects of the cytoskeleton. Additionally, please make sure you understand the process of pinocytosis and consider how cytochalasin B exposure might alter it.
Thinking ahead:
Over the course of the next two labs, you will be using direct fluorescent labeling and fluorescence microscopy to visualize the actin cytoskeleton and pinocytotic vesicles. Fluorescence microscopy is just one of many technologies that can be used to image cells and their components. To become familiar with some other imaging techniques, you and your lab partners will be asked to research a particular imaging technique and to find one research article that uses that technique to examine some aspect of the cytoskeleton. Your group will prepare a short presentation about the imaging method and its application to the cytoskeleton, which you will share with your classmates during the last lab session. You should choose an imaging technique and find an appropriate research report by your Lab 10 meeting day.
References:
Alberts, Bruce, et al. (1994) Molecular Biology of the Cell 3rded. Grand Publishing Inc., New York.
Bauer, John D., et al. (1968) Bray's Clinical Laboratory Methods 7th ed. The C.V. Mosby Co., St. Louis.
Freshney, R. Ian (1987) Culture of Animal Cells: A Manual of Basic Techniques 2nd ed. Alan R. Liss Inc., New York.
