BISC 219/F10: Lab 6: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| Line 69: | Line 69: | ||
[[BISC 219/F10: Lab 4 | Lab 4: Linkage Test Part 2, Mapping and Complementation]]<br> | [[BISC 219/F10: Lab 4 | Lab 4: Linkage Test Part 2, Mapping and Complementation]]<br> | ||
[[BISC 219/F10: Lab 5 | Lab 5: Mapping Con't]]<br> | [[BISC 219/F10: Lab 5 | Lab 5: Mapping Con't]]<br> | ||
[[BISC 219/F10: Lab 6 | Lab 6: Finish Complementation; Mapping Con't]<BR> | [[BISC 219/F10: Lab 6 | Lab 6: Finish Complementation; Mapping Con't]]<BR> | ||
[[BISC 219/F10: Lab 7 | Lab 7: Complete Mapping: Score]]<br> | [[BISC 219/F10: Lab 7 | Lab 7: Complete Mapping: Score]]<br> | ||
Series3:<BR> | Series3:<BR> | ||
Revision as of 13:41, 30 September 2010
Lab 6: Series2 Forward Genetics Project
Mapping: Count all adult and L4 progeny from one plate, scoring as either wild type , Dpy , Unc or Dpy Unc. Also score the second plate unless you have counted >100 total animals. Remember to remove each animal after you have determined its phenotype.
How would you get the single mutant class if you started with linked genes d u/+ + (genotype of the male) and d u/ d u (genotype of the hermaphrodite parent)?
You will determine map distances using the formula: RF (recombinant frequency) =the number of single mutants (both dpy and unc single mutants) divided by the total number of worms counted * 100 (to obtain RF in % recombinants and thus in map units).
Congratulations! You now have an idea about the location of the dpy mutation in map unit distance away from a reference unc gene on the a particular autosome. From your complementation analysis, you may (or may not) have found the name of the gene. To learn more, you can enter the dpygene name (if you found an allelic association through your complementation analysis) into the C. elegans database: | Wormbase at http://www.wormbase.org Wormbase OR , if none of your tested strains were allelic in complementation analysis, you can enter the name of the linked unc gene and find the genes that are your calculated number of map units away from the linked unc gene. (Remember that you will have to look in both directions on the chromosome). Is there a previously characterized dpy gene at either of those map locations? If so, you are likely to know the name of the gene associated with your dpy' mutation. Is there no previously identified dpy gene there? If not, congratulations, you may have discovered a new gene or a new function of an known gene.
Click on the link to Wormbase above and enter your dumpy gene name or your linked unc gene name into the box at the top of the page and click Search. It will either bring you directly to that page or it will bring you to a page with mutiple hits - click on the link that provides a definition for what the gene does.
On this new page should be all the known information about this particular gene. Its name, who named it, what the gene encodes - if that is known, and much more. At the bottom will be a list of references - or a link to a list of references. If you are looking at your dumpy gene information, read further. Does it appear that you are working with a well characterized gene?
Remember that even If you aren't sure of your dumpy gene name because found complementarity with all of the 4 dumpy reference strains (or other confusing complementation results), you can find out more about your dumpy gene on Wormbase from the location of the linked unc gene. You can start looking up the unc reference gene. Since you know that your dumpy gene is on the same chromosome and is a certain number of map units away from this known unc reference gene; therefore, you might can use location information about the genes around your linked unc gene to see if there is a well characterized dumpy gene in the position you have mapped it (+/- the number of map units) on that chromosome. Enter the unc reference gene name in Wormbase and click on Mapping Data. Scroll down the page until you find the mapping data for that gene and see if there is a known dumpy gene at the location you have mapped your mutation relative to this unc gene. If there is, you now know the name of your dumpy gene and you can enter that gene name in Wormbase to learn more about it. If you find no known dumpy associated gene at this map location, it is possible that you have found a new dumpy gene or a new function for an otherwise characterized gene. Your next step would be to see what is known about that location on the chromosome and see if what's known fits in at all with your observations. If it does, great and, if not, you have some thinking to do. (You will NOT write a paper about "sources of error" in your experimental design or your execution of the experiment!!!!)
Spend some time with Wormbase and marvel at all the hard work and years of research that went into discovering all this information about this tiny little nematode that causes us no harm (non-parasitic). Why do you think so many smart people have devoted so much of their time and energy to working out the genetics of "appearance or movement challenged" little worms? We will talk more about model organisms and the power of functional and comparative genomics in our next series.
Sequencing Analysis
Sequencing a portion of our genes of interest will provide us with direct evidence of the nature of the mutation (i.e: point mutation or deletion) in the mutant worms. It will help us make conclusions whether or not this mutation has been previously characterized or not. This knowledge will be useful to establish a link with the type of functional defect present in the protein produced by the mutated gene.
There are three major steps in a sequencing reaction, which are repeated for 25 cycles in a thermocycler:
- Denaturation at 94°C: During the denaturation, the double strand melts open to single stranded DNA and all enzymatic reactions stop, including the extension from the previous cycle.
- Annealing at 50°C: In each sequencing reaction, a specific primer is required. This primer is a short sequence of bases complementary to a region of the plasmid upstream of the target gene. The primer will anneal and stay, we hope, only at one position on the single stranded DNA template. In sequencing reactions there is only one strand copied whereas, in PCR, two primers are used so both strands of the DNA template are copied. The primer jiggles around, caused by the Brownian motion. Ionic bonds are constantly formed and broken between the single stranded primer and the single stranded template. The more stable bonds last a little bit longer (primers that fit exactly) and on that small section of double stranded DNA (template and primer), the polymerase can attach and starts copying the template. Once there are a few bases built in, the ionic bond is so strong between the template and the primer, that it does not break anymore.
- Extension at 60°C: Sixty degrees is the ideal working temperature for polymerase activity in sequencing even though, normally, it is 72 °C. Because the growing strand must incorporate ddNTP's which are chemically modified with a fluorescent label, the temperature is lowered so it has time to incorporate the 'strange' molecules. Well designed primers, where there are a few bases built in, already have a stronger ionic attraction to the template than the forces breaking these attractions. Primers that are on positions with no exact match, come loose again and don't give an extension of the fragment. The bases (complementary to the template) are coupled to the primer on the 3'side (adding dNTP's or ddNTP's from 5' to 3', reading from the template from 3' to 5' side, bases are added complementary to the template). When a ddNTP is incorporated, the extension reaction stops because a ddNTP contains a H-atom on the 3rd carbon atom (dNTP's contain a OH-atom on that position). Since the ddNTP's are fluorescently labeled, it is possible to detect the color of the last base of this fragment on an automated sequencer.
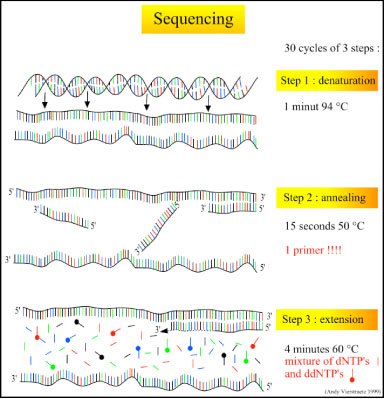
Because only one primer is used, only one strand is copied during sequencing, there is a linear (not log as in PCR) increase of the number of copies of one strand of the gene. Therefore, there has to be a large number of copies of the gene in the starting mixture for sequencing. If there are 1000 copies of the wanted gene before the cycling starts, after one cycle there will be 2000 copies: the 1000 original templates and 1000 complementary strands with each one fluorescent label on the last base. After two cycles, there will be 2000 complementary strands, three cycles will result in 3000 complementary strands and so on.

What your instructor did for you:
Due to time restraints your instructor completed the sequencing for you and it is your job to analyze the results of the sequencing reaction.
Steps in sequencing a C. elegans gene:
1. Digest the tough outer cuticle.
Add worms to a mixture of 10 mM Tris, 50 mM KCl, 1.5 mM MgCl2, pH 8.3, with 10 mg/ml Proteinase K.
Freeze the worms at -80°C for 15 minutes
Incubate at 65°C for 1 hour to digest the cuticle and then at 95°C for 15 minutes to denature the Proteinase K.
2. Amplify the gene of interest by Polymerase Chain Reaction (PCR)
Add dNTPs to a final concentration of 0.2 mM and primers to a final concentration of 0.4 mM each.
Add Taq according to manufacturer's instructions.
Determine the proper reaction conditions for your primers and gene size.
After amplification check for success via agarose gel electrophoresis.
3. Purify the product and send for sequencing
Remove all impurities and chemicals from the PCR product using a Qiagen PCR Purification Kit
following manufacturer's instructions.
Quantify DNA concentration.
Send proper concentration of DNA and primers to company for sequencing. We have used Genewiz.
What you need to do:
Assignment
Remember to check the Assignment section of the wiki for instructions about the graded assignment due in the next lab and check the Weekly Calendar for other work to accomplish before the next lab.
Links to Labs& Project Info
Series1:
Worm Info
Lab 1: Worm Boot Camp & Sex-Linked or Autosomal Start
Lab 2: Sex-Linked or Autosomal Finale
Series2:
Background: Classical Forward Genetics and Gene Mapping
Lab 2: Mutant Hunt
Lab 3: Linkage Test Part 1
Lab 4: Linkage Test Part 2, Mapping and Complementation
Lab 5: Mapping Con't
Lab 6: Finish Complementation; Mapping Con't
Lab 7: Complete Mapping: Score
Series3:
Schedule of Reverse Genetics Project
RNAi General Information
Media Recipes
Lab 5: Picking your gene to RNAi
Lab 6: Cloning your gene of interest
Lab 7: Picking your transformant
Lab 8: Plasmid purification and transformation
Lab 9: Induction of bacteria for RNAi
Lab 10: Scoring your worms and RNA purification
Lab 11: RT PCR reactions
