BISC 219/F10: Lab 6
Lab 6 : Forward Genetics: Sequencing Analysis
Once we know our gene's name or location on the genome we can sequencing a portion of our gene of interest to provide us with direct evidence of the nature of the mutation (i.e: point mutation or deletion) in the mutant worms. It will help us make conclusions whether or not this mutation has been previously characterized or not. This knowledge will be useful to establish a link with the type of functional defect present in the protein produced by the mutated gene.
There are three major steps in a sequencing reaction, which are repeated for 25 cycles in a thermocycler:
- Denaturation at 94°C: During the denaturation, the double strand melts open to single stranded DNA and all enzymatic reactions stop, including the extension from the previous cycle.
- Annealing at 50°C: In each sequencing reaction, a specific primer is required. This primer is a short sequence of bases complementary to a region of the plasmid upstream of the target gene. The primer will anneal and stay, we hope, only at one position on the single stranded DNA template. In sequencing reactions there is only one strand copied whereas, in PCR, two primers are used so both strands of the DNA template are copied. The primer jiggles around, caused by the Brownian motion. Ionic bonds are constantly formed and broken between the single stranded primer and the single stranded template. The more stable bonds last a little bit longer (primers that fit exactly) and on that small section of double stranded DNA (template and primer), the polymerase can attach and starts copying the template. Once there are a few bases built in, the ionic bond is so strong between the template and the primer, that it does not break anymore.
- Extension at 60°C: Sixty degrees is the ideal working temperature for polymerase activity in sequencing even though, normally, it is 72 °C. Because the growing strand must incorporate ddNTP's which are chemically modified with a fluorescent label, the temperature is lowered so it has time to incorporate the 'strange' molecules. Well designed primers, where there are a few bases built in, already have a stronger ionic attraction to the template than the forces breaking these attractions. Primers that are on positions with no exact match, come loose again and don't give an extension of the fragment. The bases (complementary to the template) are coupled to the primer on the 3'side (adding dNTP's or ddNTP's from 5' to 3', reading from the template from 3' to 5' side, bases are added complementary to the template). When a ddNTP is incorporated, the extension reaction stops because a ddNTP contains a H-atom on the 3rd carbon atom (dNTP's contain a OH-atom on that position). Since the ddNTP's are fluorescently labeled, it is possible to detect the color of the last base of this fragment on an automated sequencer.
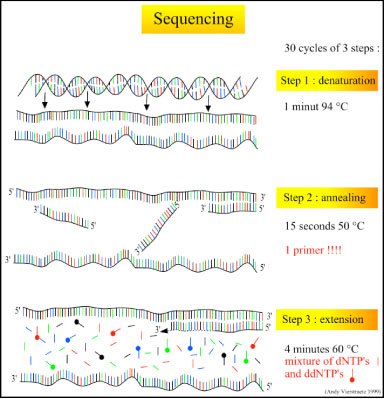
Because only one primer is used, only one strand is copied during sequencing, there is a linear (not log as in PCR) increase of the number of copies of one strand of the gene. Therefore, there has to be a large number of copies of the gene in the starting mixture for sequencing. If there are 1000 copies of the wanted gene before the cycling starts, after one cycle there will be 2000 copies: the 1000 original templates and 1000 complementary strands with each one fluorescent label on the last base. After two cycles, there will be 2000 complementary strands, three cycles will result in 3000 complementary strands and so on.

What your instructor did for you:
Due to time restraints your instructor completed the sequencing for you and it is your job to analyze the results of the sequencing reaction. One of the reasons you need to complete either the mapping or the complementation analysis before DNA sequencing of the mutated gene is that the specificity of the DNA amplification is due to the specificity of primers used. We have to know the name of the gene so that we can look up its sequence in Wormbase and design and order short sequences of DNA that will anneal and copy that gene only or a section of that gene.
Steps in sequencing a C. elegans gene:
1. Digest the tough outer cuticle.
Add worms to a mixture of 10 mM Tris, 50 mM KCl, 1.5 mM MgCl2, pH 8.3, with 10 mg/ml Proteinase K.
Freeze the worms at -80°C for 15 minutes
Incubate at 65°C for 1 hour to digest the cuticle and then at 95°C for 15 minutes to denature the Proteinase K.
2. Amplify the gene of interest by Polymerase Chain Reaction (PCR) using primers designed to amplify only this gene or a part of this gene.
Add dNTPs to a final concentration of 0.2 mM and primers to a final concentration of 0.4 mM each.
Add Taq according to manufacturer's instructions.
Determine the proper reaction conditions for your primers and gene size.
After amplification check for success via agarose gel electrophoresis.
3. Purify the product and send for sequencing
Remove all impurities and chemicals from the PCR product using a Qiagen PCR Purification Kit
following manufacturer's instructions.
Quantify DNA concentration.
Send proper concentration of DNA and primers to company for sequencing. We have used Genewiz.
What you need to do:
To analyze your sequences, you will need to work with software that can read .abi and .seq files that are generated by automatic sequencers. If there is time, your instructor will show you our ABI automatic sequencer and explain its operation. The directions for using the DNAstar software by Lasergene to analyze your sequences are found at:
Mapping Continued: Making the males to be able to set up a test cross
You will transfer 3-5 L4 hermaphrodite true breeding double mutants to 2 separate cross plates and add 3-5 N2 (WT) males to each plate in order to create male heterozygotes (++/ d u) that we can cross with our double mutants to perform a test cross in 3 days that we will score in the next lab. Label your plates with your Matting color Sharpie. Incubate your worms at 23C for 3 days.
To make sure you have fertile L4 double mutant hermaphrodites for your next cross, transfer a single double mutant from your true breeding plate to each of 2 separate maintance plates. Label your plates with your mating project color Sharpie. Incubate at 23 C for 3 days.
TO do in 3 days
FINALLY!!! Set up the crucial TEST CROSS: Return to lab and transfer 3-5 L4 double mutant hermaphrodites to each of 2 crossing plates. You will find the clean crossing plates in your lab day's box in the supply area in the back of the lab. Add 3-5 of the heterozygous males (phentypically wild type) of genotype (++/ d u) that you created in your last cross to each of those cross plates with the double mutant hermaphrodites. Incubate all worms at 23C until your next lab period.
Assignment
Remember to check the Assignment section of the wiki for instructions about the graded assignment due in the next lab and check the Weekly Calendar for other work to accomplish before the next lab.
Links to Labs& Project Info
Series1:
Worm Info
Lab 1: Worm Boot Camp & Sex-Linked or Autosomal Start
Lab 2: Sex-Linked or Autosomal Finale
Series2:
Background: Classical Forward Genetics and Gene Mapping
Lab 2: Mutant Hunt
Lab 3: Linkage Test Part 1
Lab 4: Linkage Test Part 2, Mapping and Complementation
Lab 5: Finish Complementation; Mapping Con't
Lab 6: DNA sequence analysis; Mapping Con't
Lab 7: Complete Mapping: Score
Series3:
Schedule of Reverse Genetics Project
RNAi General Information
Media Recipes
Lab 5: Picking your gene to RNAi
Lab 6: Cloning your gene of interest
Lab 7: Picking your transformant
Lab 8: Plasmid purification and transformation
Lab 9: Induction of bacteria for RNAi
Lab 10: Scoring your worms and RNA purification
Lab 11: RT PCR reactions
