BME100 s2015:Group12 12pmL3: Difference between revisions
(→Graph) |
|||
| (8 intermediate revisions by the same user not shown) | |||
| Line 83: | Line 83: | ||
==Target Population and Need== | ==Target Population and Need== | ||
This device targets the 18-45 year old demographic in the United States. Such a selection is based on the belief that men and women of ages within this group are at the highest risk of STD exposure. | |||
Due to the astronomical amount of total individuals who have potentially been exposed to some sort of sexually transmitted disease, the Phalanx Monitor targets the five most commonly known STD’s in American society: HIV, Syphilis, Gonorrhea, Chlamydia and Herpes. It is rather difficult to pinpoint the exact number of people the device will affect. However, the following values may possibly give more clarity on how many people the device will target generally: | Due to the astronomical amount of total individuals who have potentially been exposed to some sort of sexually transmitted disease, the Phalanx Monitor targets the five most commonly known STD’s in American society: HIV, Syphilis, Gonorrhea, Chlamydia and Herpes. It is rather difficult to pinpoint the exact number of people the device will affect. However, the following values may possibly give more clarity on how many people the device will target generally: | ||
| Line 110: | Line 109: | ||
Needs of the population: | Needs of the population: | ||
Affordability: It is well understood that since the worldwide financial disaster of 2008, money has been a major issue for the majority of the American population. Everyone has been affected in some way since then. With the prevalence of sexually transmitted diseases in the nation, high quality STD testing comes at a price. | Affordability: It is well understood that since the worldwide financial disaster of 2008, money has been a major issue for the majority of the American population. Everyone has been affected in some way since then. With the prevalence of sexually transmitted diseases in the nation, high quality STD testing comes at a price. We aim to offer the best quality product at a price which is made to be the most affordable to the population, $49.99. If people are unable to purchase an STD test, they are more susceptible to inaction, allowing the disease to more effectively ravage their bodies. | ||
Accessibility: Being able to purchase the product directly from the company’s website and phone number shows that the device is not difficult to acquire. People, especially the younger age groups in the demographic targeted, have trouble being able to figure out where to go to get the | Accessibility: Being able to purchase the product directly from the company’s website and phone number shows that the device is not difficult to acquire. People, especially the younger age groups in the demographic targeted, have trouble being able to figure out where to go to get the tools they need to amend their condition. The company aims to simplify accessibility in order to make certain that our device can reach those who need it. | ||
Accuracy: No amount of testing can erase the critical nature of data accuracy. If an individual suspects they may have contracted an STD, they want to be sure that if they complete an STD test, they want the disease identified as close to 100% of the time as possible. Our device is estimated to have a nearly 99% accuracy level for identifying the appropriate STD's from the person’s saliva, comparable to the accuracy of blood and urine tests. | Accuracy: No amount of testing can erase the critical nature of data accuracy. If an individual suspects they may have contracted an STD, they want to be sure that if they complete an STD test, they want the disease identified as close to 100% of the time as possible. Our device is estimated to have a nearly 99% accuracy level for identifying the appropriate STD's from the person’s saliva, comparable to the accuracy of blood and urine tests. | ||
| Line 125: | Line 124: | ||
NOTE: The author of this section was unable to successfully label the dimensions of the device with Solidworks 2013. | NOTE: The author of this section was unable to successfully label the dimensions of the device with Solidworks 2013. | ||
Dimensions will have to be typed manually here. | Dimensions will have to be typed manually here. | ||
The Phalanx Monitor scans the saliva of an individual to see if the person has been exposed to a sexually transmitted disease (STD) covered by the device. This is to eliminate the more invasive or strenuous methods of STD testing such as blood, urine, vaginal or seminal fluid tests. | |||
| Line 232: | Line 235: | ||
[[Image:ChlamydiaData.jpg]] | [[Image:ChlamydiaData.jpg]] | ||
[[Image:PhalanxAccuracy.jpg]] | |||
<br><br> | <br><br> | ||
| Line 247: | Line 255: | ||
[[Image:ChlamydiaDataGraph.jpg]] | [[Image:ChlamydiaDataGraph.jpg]] | ||
[[Image:PhalanxCompatible.jpg]] | |||
To clarify the graph above, the compatibility percentages represent how accurate the Phalanx Monitor's saliva tests are when | |||
compared to the gold standards of blood and urine tests. | |||
<br><br> | <br><br> | ||
Revision as of 22:46, 3 March 2015
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
|
OUR TEAM
LAB 3A WRITE-UPDescriptive StatisticsResultsTemperature Results Between a Thermometer and the Spree Headband
AnalysisThe team studied the effectiveness of the Spree headband compared to the thermometer and the Pulse Ox device which both served as gold standards for measuring the heart beats per minute and the temperature of a subject respectively. Data analysis showed that the Pearson's Correlation Coefficient of -0.053072216 for temperature represents a negative correlation between the Spree headband and the thermometer. The temperature's T-Test value of 8.5316E-20 is less than the alpha value of 0.05, which is used as a standard to measure data significance. This indicates that there was a significant difference in data between the headband and the thermometer. Based on these two factors, the team believes that the headband was insufficient in measuring the body temperature of an individual accurately. However, the team's data for measuring the rate of heart beats per minute gives a different conclusion. The Pearson's Correlation Coefficient of 0.51788901 indicates that there is a positive correlation between the Spree headband and the Pulse Ox that was used as a gold standard. This discovery is reinforced by the T-Test value of 0.116138892 that was also determined. Since this value surpasses the standard alpha value of 0.05, the conclusion that there was no significance in data between the two temperature groups can be made. This may suggest that data accuracy for measuring the heart beats per minute in an individual may be more apparent, giving the Spree headband more legitimacy in that area of expertise.
Summary/DiscussionBefore the team gives their opinion on the Spree headband device, a disclaimer regarding the experiment must be made. The team's study of the device was postponed by forty-five minutes of time. There was an insufficient amount of Spree devices provided for the laboratory and the supervisors of the lab were forced to find more. This large gap in available time for the experiment proved a hindrance on data collection. The team personally was only able to accomplish half of the experiment with the remaining time left, which may have played some sort of role in the conclusions made regarding the Spree device.
With the aforementioned information above, the Spree headband device was an attempt of being a multipurpose fitness tool capable of providing any major needs an individual would have in those activities. It was a failure for several reasons. First, the device required consistent motion on the individual's part in order for data collection to occur. Placed on the forehead, the individual would be less likely to move their head compared to other body parts, such as the wrist or the leg. The team believes that by relocating the device to a more active area on the body, the Spree device would have had better data accuracy and overall reliability, if motion does have to be a factor here. Second, the stress the Spree headband placed on the battery life of a mobile smartphone seemed to be too demanding. One of the research team's members used their Apple iPhone for data collection. The iPhone started with a charge of 35%. By the conclusion of the experiment, the phone was down to a charge of 7% after one hour of use. The team was forced to end the experiment at this moment. They understood that mobile Apple products have a failsafe battery shutdown at 5%, to prevent the death of the mobile's battery. The team recommends that more measures are made to improve the battery demand of the Spree device in order to justify its use to begin with. Lastly, the data collection and how it was represented by the Spree headband was not as simple as the company had advertised. The temperature of an individual was measured using a color and level code. On the device, blue was level 1, yellow was level 2, a partially filled red bar was level 3, and a fully red bar was level 4. No actual numerical measure was given for temperature, which required the team to decipher what each level meant. This system of measuring temperature is ineffective in providing what the customer would want when recording body temperature. The team recommends the removal of the color and level code and replacing it by using both the Fahrenheit and Celsius numerical method. This is to provide a more simplistic interaction with the Spree device and the individual who uses it.
LAB 3B WRITE-UPTarget Population and NeedThis device targets the 18-45 year old demographic in the United States. Such a selection is based on the belief that men and women of ages within this group are at the highest risk of STD exposure. Due to the astronomical amount of total individuals who have potentially been exposed to some sort of sexually transmitted disease, the Phalanx Monitor targets the five most commonly known STD’s in American society: HIV, Syphilis, Gonorrhea, Chlamydia and Herpes. It is rather difficult to pinpoint the exact number of people the device will affect. However, the following values may possibly give more clarity on how many people the device will target generally:
Herpes: Approximately 24 million Americans affected HIV: Approximately 900,000 Americans affected Chlymydia: Approximately 1.4 million Americans affected Gonorrhea: Appproximately 800,000 Americans affected
Affordability: It is well understood that since the worldwide financial disaster of 2008, money has been a major issue for the majority of the American population. Everyone has been affected in some way since then. With the prevalence of sexually transmitted diseases in the nation, high quality STD testing comes at a price. We aim to offer the best quality product at a price which is made to be the most affordable to the population, $49.99. If people are unable to purchase an STD test, they are more susceptible to inaction, allowing the disease to more effectively ravage their bodies. Accessibility: Being able to purchase the product directly from the company’s website and phone number shows that the device is not difficult to acquire. People, especially the younger age groups in the demographic targeted, have trouble being able to figure out where to go to get the tools they need to amend their condition. The company aims to simplify accessibility in order to make certain that our device can reach those who need it. Accuracy: No amount of testing can erase the critical nature of data accuracy. If an individual suspects they may have contracted an STD, they want to be sure that if they complete an STD test, they want the disease identified as close to 100% of the time as possible. Our device is estimated to have a nearly 99% accuracy level for identifying the appropriate STD's from the person’s saliva, comparable to the accuracy of blood and urine tests. Privacy: The history of sexuality in America has consistently been of a reclusive and conservative nature. Despite the amount of advertisement readily available in numerous media outlets, the American population is reluctant to have an open and honest discussion regarding sexual intercourse, outercourse or any sort of similar activity. Having to purchase and use an STD test can be humiliating and embarrassing for people who do not want to disclose these kinds of problems. At our company, the purchase and use of our device demands the absolute amount of privacy an individual has rights to. The device can be purchased at our company website or phone number with confidentiality. Products shipped to the homes do not advertise the product itself. Only the person who bought the device knows what is in the package that arrives at their door. The device can also be shipped to a specified address if the person does not want their family, relatives or friends to find out that they have purchased the device. Hospitals, health clinics, school and university health services are excellent examples of product acquirement with total privacy, as the shipping address can be specified with the purchase.
Device DesignNOTE: The author of this section was unable to successfully label the dimensions of the device with Solidworks 2013. Dimensions will have to be typed manually here.
The Phalanx Monitor scans the saliva of an individual to see if the person has been exposed to a sexually transmitted disease (STD) covered by the device. This is to eliminate the more invasive or strenuous methods of STD testing such as blood, urine, vaginal or seminal fluid tests.
This is the overview of the Phalanx Monitor for sexually transmitted diseases. DIMENSIONS: 6 inches length * 6 inches width * 6 inches height = 216 cubic inches. Each face of the device is 6 inches in length by 6 inches in height. Weight: 5 pounds (lbs) This is the front view of the Phalanx Monitor. Here is where the Phalanx Saliva Cartridge is collected for disposal after testing. DIMENSIONS: Phalanx Saliva Cartridge exit port: 1 inch in length, 0.5 inches in height, 4 inches in depth. This is the top side of the Phalanx Monitor. The USB cord for either the Android or Apple smartphone is plugged into here so data can be uploaded into the phone via app. DIMENSIONS: USB port: 0.5 inches in length, 0.25 inches in height, 0.5 inches in depth This is the bottom side. Two double AA batteries will be placed into here to power the Monitor. DIMENSIONS: 2.2 inches in length, 2.2 inches in height, 0.3 inches in depth. This is the left side of the device. The switch to either turn on or off the Phalanx Monitor is here. DIMENSIONS: 1 inch in length, 1 inch in height, 0.3 inches in depth. This is the right side of the device. The entry port is here for the Phalanx Saliva Cartridge. DIMENSIONS: Phalanx Saliva Cartridge entry port: 2 inches in length, 0.5 inches in height, 3.5 inches in depth. This is the Phalanx Saliva Cartridge. There is where the saliva of the subject will be placed into for data analysis. DIMENSIONS: 2 inches in length, 0.5 inches in height, 1 inch in width.
How The Device Works: - The customer connects the device to their smartphone via USB cord. - The Phalanx Monitor app is to be downloaded onto their phone. - Two AA batteries are placed into the device on the bottom side of the Monitor. - The switch on the left side of the Phalanx Monitor is flipped to the On position. - The person will place a sample of their saliva into a Phalanx Saliva Cartridge. - The person will then insert horizontally the cartridge containing their saliva into the Phalanx Monitor entry port on the right side of the device. - Mechanically, the cartridge will be moved to the position just above the analytical sensor in the internal center of the device. - The analytical sensor will perform a quick sweep of the space just above it and will determine if there is a cartridge that contains the saliva sample. - From here, the Phalanx Monitor's sensor will perform a deep, thorough scan of the saliva sample. The sensor is designed to avoid the physical properties of the cartridge, which makes the sensor believe the saliva sample is "suspended in open space," preventing the cartridge from affecting the results. - The scan will take up to five minutes to complete and will display the data on the person's smartphone via USB cord. - The test results will show what STD's the person is positive and negative for. The diseases have informational databases programmed into the app, allowing the individual to better understand their condition and serves as a source of education. This was done to potentially reduce the fear factor of sexually transmitted diseases. - Once the scan is complete, the saliva cartridge will be discharged vertically in a manner similar to a floppy disk, where the individual will then dispose of the cartridge appropriately. This could be done on the front side of the Phalanx Monitor.
Inferential Statistics
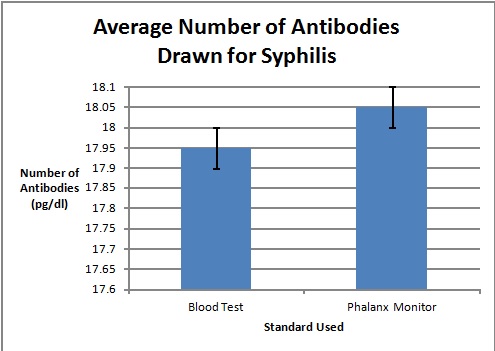
Graph
To clarify the graph above, the compatibility percentages represent how accurate the Phalanx Monitor's saliva tests are when compared to the gold standards of blood and urine tests.
|
||||||