BME103:T130 Group 2: Difference between revisions
| (42 intermediate revisions by 2 users not shown) | |||
| Line 13: | Line 13: | ||
{| style="wikitable" width="700px" | {| style="wikitable" width="700px" | ||
|- valign="top" | |- valign="top" | ||
| [[Image: | | [[Image:lexie101.jpg|100px|thumb|Name: Alexandra Brunelle<br>Research and Development Specialist]] | ||
| [[Image:BME103_group2_12.jpg|100px|thumb|Name: Charul Singh<br>Experimental Protocol Planner]] | | [[Image:BME103_group2_12.jpg|100px|thumb|Name: Charul Singh<br>Experimental Protocol Planner]] | ||
| [[Image:BME103_Group2_AlexHoffmann.jpg|100px|thumb|Name: Alex Hoffmann<br>Experimental Protocol Planner]] | | [[Image:BME103_Group2_AlexHoffmann.jpg|100px|thumb|Name: Alex Hoffmann<br>Experimental Protocol Planner]] | ||
| [[Image:AJ101.jpg|100px|thumb|Name: AJ Ciferno<br>Open PCR Machine Engineer]] | | [[Image:AJ101.jpg|100px|thumb|Name: AJ Ciferno<br>Open PCR Machine Engineer]] | ||
| [[Image:Dog.jpeg|100px|thumb|Name: Haley Gjertsen<br>Research and Development Specialist]] | | [[Image:Dog.jpeg|100px|thumb|Name: Haley Gjertsen<br>Research and Development Specialist]] | ||
| | | | ||
|} | |} | ||
| Line 27: | Line 27: | ||
'''The Original Design'''<br> | '''The Original Design'''<br> | ||
[[Image: | [[Image:HALEY.png]]<br><br> | ||
The Polymerase Chain Reaction (PCR) machine is used to test for variations in nucleotides. It is simple, easy to use, and portable. For our purposes we used the PCR machine to test for cancer in patients. It was able to test up to sixteen test tubes at a time and took slightly more than an hour and a half to complete the cycles. It heats up in order to separate the DNA strand and then cools to allow primers to attach and new replicated strands to form. It then heats back up to repeat the same cycles. | |||
| Line 35: | Line 37: | ||
When we unplugged the LCD screen from the PCR machine, the machine's display screen stopped working, but information continued to transmit to the computer. | When we unplugged the LCD screen from the PCR machine, the machine's display screen stopped working, but information continued to transmit to the computer. | ||
When we unplugged the white wire that connects the Thermocouple to the 16-tube PCR block, the machine's temperature measurement was disabled | When we unplugged the white wire that connects the Thermocouple to the 16-tube PCR block, the machine's temperature measurement was disabled, in other words, it no longer kept track of heat readings. | ||
'''Test Run'''<br> | '''Test Run'''<br> | ||
On October 18th and 20th the PCR machine responded normally with no signs of malfunction. However, it took a little bit longer to complete the cycle; specifically, it took an hour and forty minutes in total. The machine LED display matched the computer screen which was a sign of success and proper function. | |||
| Line 51: | Line 53: | ||
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
|+'''Polymerase Chain Reaction''' | |+'''Polymerase Chain Reaction'''<br> | ||
! '''Reagent'''!! '''Volume''' | ! '''Reagent'''!! '''Volume''' | ||
|- | |- | ||
| Line 71: | Line 73: | ||
<br> | <br> | ||
'''Sample Descriptions''' | '''Sample Descriptions''' | ||
<br> | <br> | ||
| Line 99: | Line 100: | ||
'''Set up PCR Proceedures:''' | '''Set up PCR Proceedures:''' | ||
<br> | <br> | ||
1. Label each tube differently and record label.<br> | |||
2. Insert reactants into their respective PCR tubes.<br> | |||
3. Connect assembled PCR machine to a computer.<br> | |||
4. Customize settings in program to two stages: <br> | |||
Stage 1: 1 cycle, heat to 95 degrees Celsius for 3 minutes<br> | |||
Stage 2: 35 cycles, heat to 95 degrees Celsius for 30 seconds; cool to 57 degrees Celsius for 30 seconds<br> | |||
Stage 3: heat to 72 degrees Celsius for 30 seconds.<br> | |||
5. Start up the OpenPCR program previously installed on the computer.<br> | |||
6. Place tubes in slots in PCR machine, and close lid tightly.<br> | |||
7. Lower the top of the lid until it just touches tops of tubes.<br> | |||
8. Press "Start" to run the PCR machine. <br> | |||
9. Record data.<br> | |||
<br> | <br> | ||
'''Using a Fluorimeter'''<br> | |||
'''Using a | |||
[[Image: | [[Image:PCR101.png]]<br><br> | ||
1. Smart Phone Stand<br> | |||
2. Smart Phone<br> | |||
3. Glass Slide and Blue Light Holder<br> | |||
4. Box | |||
<br><br> | <br><br> | ||
''' | '''Fluorimeter Assembly Procedures:''' | ||
1. Place the slide (glass facing down) onto the device.<br> | <br> | ||
1. Adjust the settings on the smart phone: no flash, contrast on the lowest setting, saturation on the highest setting and exposure on the highest setting<br> | |||
2. Place the slide (glass facing down) onto the device.<br> | |||
3. Using the pipette for SYBR Green Flourescent Dye, place two drops of the dye onto the center line two dots.<br> | |||
4. Now, using a pipette that corresponds to a specific solution, place two drops of the given solution on top of the SYBR Green drops.<br> | |||
5. Switch on the blue light and adjust slide to allow the light to shine through the solution drops.<br> | |||
6. Place the phone in the holder facing the device. (should be about two inches away)<br> | |||
7. Cover the device and the phone and holder with the black box to keep out light. <br> | |||
8. Take picture. For best results, set a timer on the phone to be able to close the box and maximize darkness.<br> | |||
9. Using the pipette marked for waste, remove the drops from the slide.<br> | |||
10. Repeat steps 3 through 8 for all samples, each time moving down the slide. You can conduct five tests per slide; in other words you will need two slides total.<br><br> | |||
'''ImageJ Set Up Procedures:'''<br> | '''ImageJ Set Up Procedures:''' | ||
<br> | |||
1. Download ImageJ onto a laptop using the ImageJ disk.<br> | 1. Download ImageJ onto a laptop using the ImageJ disk.<br> | ||
2. Save desired photos to desk top.<br> | 2. Save desired photos to desk top.<br> | ||
3. | 3. Open ImageJ <br> | ||
4. On the menu, select ANALYZE, and then select SET MEASUREMENTS. Now choose Area, Integrated Density, and Mean Grey Value.<br> | |||
5. Now you can upload photo to ImageJ<br> | |||
6. In order to analyze the pictures, you need to make the menu selection IMAGE. Now select COLOR. Finally, choose SPLIT CHANNELS. This will bring up three windows labeled blue, green, and red. Since SYBR green dye is green, you will want to analyze the photos under the window marked Green. Close the windows marked Red and Blue.<br> | |||
7. Now, using the oval tool, draw an oval around the drop and select ANALYZE and then select MEASURE. Write down the data given by ImageJ. <br> | |||
8. Using your laptops arrow keys, move the oval to a dark area in the picture to get data for the "noise." Again, select ANALYZE and then select MEASURE. Write down the data given by ImageJ.<br> | |||
9. Repeat steps 5 through 8 for all of the images. <br> | |||
==Research and Development== | ==Research and Development== | ||
| Line 143: | Line 157: | ||
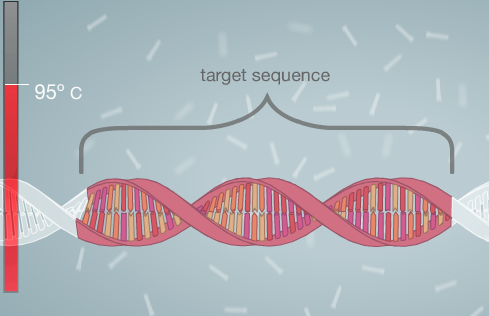
'''PCR stands for Polymerase Chain Reaction.''' This reaction is used to amplify a specific segment of DNA that codes for cancer.<br><br> | '''PCR stands for Polymerase Chain Reaction.''' This reaction is used to amplify a specific segment of DNA that codes for cancer.<br><br> | ||
[[Image: | [[Image:DNA.png]] | ||
'''Some basic components involved in a PCR reaction are:''' | |||
'''Template DNA:''' the original strand of DNA that is going to be copied<br> | '''Some basic components involved in a PCR reaction are:'''<br> | ||
'''Primers:''' attach to the sites on the DNA strands that are at either end of the template DNA | '''Template DNA:''' This DNA template is the original strand of DNA that is going to be copied<br> | ||
'''Taq Polymerase:''' reads the DNA code and matches new nucleotides with the template strand | '''Primers:''' These primers attach to the sites on the DNA strands that are at either end of the template DNA. They drive the PCR because they are powerful enzymes that allow replication to occur. In this reaction, if primers are able to attach to the DNA strand, the patient is negative for cancer.<br> | ||
'''Magnesium Chloride:''' a three atom molecule that binds to Taq | '''Taq Polymerase:''' Tag Polymerase reads the DNA code and matches new nucleotides with the template strand. It bind the pairs together with hydrogen bonds, for example A's are bonded to T's and C's are bonded to G's. These base pairs make it possible to make several copies of the DNA strands. <br> | ||
'''dNTP's:''' | '''Magnesium Chloride:''' Magnesium Chloride is a three atom molecule that binds to Taq Polymerase as a co-factor to help Tag Polymerase it function properly.<br> | ||
'''dNTP's:''' Deoxinucleotide Triphosphates are the new nucleotides<br><br> | |||
'''A PCR functions by going through a series of thermal cycles:'''<br> | '''A PCR functions by going through a series of thermal cycles:'''<br> | ||
In the first cycle the PCR machine heats up to 95 degrees Celsius. This unzips the DNA strand creating two separate DNA strand molecules and exposes the nucleotides we wish to look at. In the second cycle the temperature drops to 57 degrees Celsius which allows the | In the first cycle, the PCR machine heats up to 95 degrees Celsius. This unzips the DNA strand creating two separate DNA strand molecules and exposes the nucleotides that we wish to look at. In the second cycle, the temperature drops to 57 degrees Celsius which allows the primers to bind to the ends of the DNA strands. The third cycle heats up the DNA to 72 degrees Celsius to allow DNA replication by the enzyme Taq Polymerase. These three cycles are repeated many more times, allowing for billions of DNA copies to be made. We need multiple copies of the DNA so that we can see if the patient tests positive for cancer.<br><br> | ||
'''r17879961'''<br> | '''r17879961'''<br> | ||
The single nucleotide polymorphism, or SNP, that codes for cancer is AACTCTTACA'''C'''TCGATACAT; in a normal patient, the C is replaced with T. The reason the C causes cancer in a patient is because there is an improper base pair | The single nucleotide polymorphism, or SNP, that codes for cancer is AACTCTTACA'''C'''TCGATACAT; in a normal patient, the '''C''' is replaced with '''T'''(AACTCTTACA'''T'''TCGATACAT). The backwards part to these two sequences is TTGAGAATGT'''A'''AGCTATAGTA. The reason the C causes cancer in a patient is because there is an improper base pair causing the primers not to bind. This results in the formation of single strands. Keep in mind that C does not code wit A, it only codes with C. After the PCR's thermal cycles are finished the cancerous patient's DNA will be single stranded and we will be able to see this after SYBR green dye is added and the sample appears green. The SYBR green dye does not express the double stranded DNA, non-cancerous patients. <br><br> | ||
'''Reliability: Bayes Rule:'''<br> | |||
We saw that the SNP is two hundred base pairs away. This means that every two hundred base pairs the missense mutation is present. <br> | |||
Bayes' Theorem demonstrates the ratio of patients with true positive and negative results to patients with false positive and negative results. It takes into account several factions of the tested population. Because of the numerous false positive and negative results, this test is not feasible for widespread usage. <br><br> | |||
Bayes Theorem is represented by the equation: p(CIT)=[p(TIC)*p(C)]/[(p(TIC)*p(C))+(p(TI~C)*p(~C))]<br><br> | |||
In our case, the variables are assigned as follows:<br> | |||
C= cancer present <br> | |||
T= positive test <br> | |||
p(C)= Prior Probability <br> | |||
p(TIC)= Conditional Probability <br> | |||
p(TI~C)= Conditional Probability<br> | |||
p(~C)= Prior Probability<br> | |||
p(CIT)= Posterior Probability <br><br> | |||
We know that:<br> | |||
p(C)= 0.01 <br> | |||
p(TIC)= 0.8 <br> | |||
p(TI~C)= 0.096 <br> | |||
p(~C)= 0.99<br><br> | |||
Therefore, <br> | |||
p(CIT)= 0.078 or 7.8%<br> | |||
'''Bonus!!'''<br> | |||
'''Below is a visual representation of what happens during a Polymerase Chain Reaction:''' <br> | |||
[[Image:DNA1.png]] <br> | |||
'''1. The DNA is heated up to 95 degrees Celsius, splitting the strand apart.'''<br> | |||
[[Image:Dna.png]]<br> | |||
'''2.The solution is cooled down to 50 degrees Celsius and primers are able to attach to the separated strands.'''<br> | |||
[[Image:OKAY2.png]]<br> | |||
'''3.The solution is heated to 72 degrees Celsius and taq Polymerase attaches.'''<br> | |||
[[Image:OKAY3.png]]<br> | |||
'''4. The DNA strands are replicated at 72 degrees Celsius.'''<br> | |||
[[Image:CHELSEA.png]]<br> | |||
'''5. The process repeats as the PCR machine heats up to 95 degrees.'''<br> | |||
<br><br> | <br><br> | ||
(Photo Credit: The images used here were borrowed from OpenPCR.) | |||
| Line 209: | Line 248: | ||
| '''Sample''' || '''Integrated Density''' || '''DNA μg/mL''' || '''Conclusion''' | | '''Sample''' || '''Integrated Density''' || '''DNA μg/mL''' || '''Conclusion''' | ||
|- | |- | ||
| PCR: Negative Control || | | PCR: Negative Control || 76818 || .064762057 || Negative | ||
|- | |- | ||
| PCR: Positive Control || | | PCR: Positive Control || 2121336 || 1.788410055 || Positive | ||
|- | |- | ||
| PCR: Patient 1 ID 74013, rep 1 || | | PCR: Patient 1 ID 74013, rep 1 || -57048 || -.048094793 || Negative | ||
|- | |- | ||
| PCR: Patient 1 ID 74013, rep 2 || | | PCR: Patient 1 ID 74013, rep 2 || 1436979 || 1.211457163 || Positive | ||
|- | |- | ||
| PCR: Patient 1 ID 74013, rep 3 || | | PCR: Patient 1 ID 74013, rep 3 || 2803635 || 2.363627933 || Positive | ||
|- | |- | ||
| PCR: Patient 2 ID 72825, rep 1 || | | PCR: Patient 2 ID 72825, rep 1 || 140996 || .118867857 || Negative | ||
|- | |- | ||
| PCR: Patient 2 ID 72825, rep 2 || | | PCR: Patient 2 ID 72825, rep 2 || 74969 || .063203242 || Negative | ||
|- | |- | ||
| PCR: Patient 2 ID 72825, rep 3 || | | PCR: Patient 2 ID 72825, rep 3 || 2880430 || 2.4283706 || Positive | ||
|} | |} | ||
KEY | KEY | ||
* '''Sample''' = | * '''Sample''' = The sample is the DNA taken from the patient in a tube. | ||
* '''Integrated Density''' = < | * '''Integrated Density''' = Integrated Density is an extensive quantity. It is the sum of the values of the pixels in the image or in the selected part of the image. This sum is the same as the product of the mean gray value and the area.<br> | ||
* '''DNA μg/mL''' = | Integrated density(Background)-Integrated density(sample)=our data | ||
* '''Conclusion''' = | * '''DNA μg/mL''' = This was calculated by multiplying the integrated density by two and dividing it by the integrated density of the calf thymus. | ||
* '''Conclusion''' = A positive signal allowed the sample to appear green. No signal resulted in the sample appearing clear. <br><br> | |||
[[Image:Poop.png]]<br> | [[Image:Poop.png]]<br> | ||
Cancerous Drop | Cancerous Drop<br><br> | ||
[[Image:Omg.png]]<br> | |||
Non Cancerous Drop<br><br> | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
|} | |} | ||
Latest revision as of 21:41, 14 November 2012
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAMLAB 1 WRITE-UPInitial Machine TestingThe Polymerase Chain Reaction (PCR) machine is used to test for variations in nucleotides. It is simple, easy to use, and portable. For our purposes we used the PCR machine to test for cancer in patients. It was able to test up to sixteen test tubes at a time and took slightly more than an hour and a half to complete the cycles. It heats up in order to separate the DNA strand and then cools to allow primers to attach and new replicated strands to form. It then heats back up to repeat the same cycles.
Experimenting With the Connections When we unplugged the LCD screen from the PCR machine, the machine's display screen stopped working, but information continued to transmit to the computer. When we unplugged the white wire that connects the Thermocouple to the 16-tube PCR block, the machine's temperature measurement was disabled, in other words, it no longer kept track of heat readings.
On October 18th and 20th the PCR machine responded normally with no signs of malfunction. However, it took a little bit longer to complete the cycle; specifically, it took an hour and forty minutes in total. The machine LED display matched the computer screen which was a sign of success and proper function.
Protocols
Sample 1: Positive Control (contains cancer DNA template), Tube label: P
Using a Fluorimeter
Fluorimeter Assembly Procedures:
ImageJ Set Up Procedures:
Research and DevelopmentSpecific Cancer Marker Detection - The Underlying Technology
r17879961
Results
Integrated density(Background)-Integrated density(sample)=our data
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||