BME103:T930 Group 3
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAM
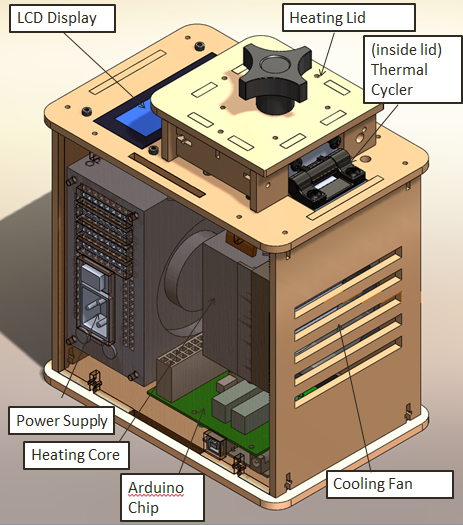
LAB 1 WRITE-UPInitial Machine TestingThe Original Design The OpenPCR is a machine that is used to replicate DNA in order to amplify a specific gene. This machine primarily uses changes in temperature and various enzymes to facilitate the replication process multiple times.
When we unplugged (part 3) from (part 6), the screen turned off. Everything on the PCR was working fine expect there was no output on the display. When we unplugged the white wire that connects (part 6) to (part 2), the reading from the screen dropped to -40 degrees Celsius.
(Write the date you first tested Open PCR and your experience(s) with the machine)
ProtocolsPolymerase Chain Reaction Polymerase Chain Reaction Procedure: 2.)We labeled 8 empty PCR tubes. For the first sample we labeled the 3 DNA samples 1A, 1B and 1C. For the second sample we labeled the tubes 2A, 2B and 2C. For the positive and negative controls, we labeled the tubes + and - respectively. 3.)Using one pipette per sample, to avoid contamination, we transferred the PCR reaction mix we were given to the PCR tubes. 4.)We then placed the samples in the PCR machine 5.)We set our PCR program to three stages. Stage one: 1 cycle, 95 degree Celsius for 3 minutes. Stage 2: 35 cycles, 95 degrees Celsius for 30 seconds, 57 degrees Celsius for 30 seconds, 72 degrees Celsius. Stage three: 72 degrees Celsius for 3 minutes and then hold at 4 degree Celsius. Fluorimeter assembly Procedure:
Research and DevelopmentSpecific Cancer Marker Detection - The Underlying Technology (Add a write-up of the information discussed in Week 3's class) (BONUS points: Use a program like Powerpoint, Word, Illustrator, Microsoft Paint, etc. to illustrate how primers bind to the cancer DNA template, and how Taq polymerases amplify the DNA. Screen-captures from the OpenPCR tutorial might be useful. Be sure to credit the source if you borrow images.)
Results
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||