BME103:T930 Group 6 l2: Difference between revisions
(New page: {|{{table}} width="800" |- |style="background-color: #EEE"|128px<span style="font-size:22px;"> BME 103 Fall 2012</span> |style="background-color: #F2F2F2" | ...) |
|||
| (39 intermediate revisions by 5 users not shown) | |||
| Line 12: | Line 12: | ||
{| style="wikitable" width="700px" | {| style="wikitable" width="700px" | ||
|- | |- valign="top" | ||
| [[Image: | | [[Image:Snapshot_20121114.JPG|100px|thumb|Name: Nicholas Sterkowitz<br>Open PCR machine engineer]] | ||
| [[Image: | | [[Image:D.jpg|100px|thumb|Name: Dominic Ilardi<br>Open PCR machine engineer]] | ||
| [[Image: | | [[Image:new_york.jpg|100px|thumb|Name: Alexandra Nazareno<br>Experimental Protocol Planner]] | ||
| [[Image: | | [[Image:BME103_Group6_Assembly.jpg|100px|thumb|Name: Amanda Sweig<br>Experimental Protocol Planner]] | ||
| [[Image: | | [[Image:Taylor.jpg|100px|thumb|Name: Taylor Deegan<br>Research and Development(s)]] | ||
|} | |} | ||
| Line 25: | Line 25: | ||
==Thermal Cycler Engineering== | ==Thermal Cycler Engineering== | ||
[[Image:Aluminum_body_and_lid.png|300px|thumb|Aluminum frame instead of wood]] | |||
'''System Design'''<br> | '''System Design'''<br> | ||
Our re-design is based upon the [http://openpcr.org Open PCR] system originally designed by Josh Perfetto and Tito Jankowski. The wooden frame is changed to aluminum, an additional row and column will be added to the heating block making it 5 by 5, and there will be handles added to the sides of the machine.<br> | |||
[[Image:Handle_for_Case.png|300px|thumb|The side panel the handle will be added to]] | |||
'''Key Features'''<br> | '''Key Features'''<br> | ||
To begin with we will change the frame from a wooden construction to recycled aluminum. This will maintain the light-weight portability of the machine, but will greatly reduce the fire hazard of heating a wooden box to the required temperatures. Also, by eliminating the need for wood, the machine will be more eco-friendly. We will then add an additional row and column to the heating block to increase the product output without too much change to the overall size or cost of the machine. However, the increased productivity of the additional row and column will require the addition of a new larger heating lid. | |||
We will also add two carrying handles to either side of the machine. This will aid in the transport and increase the machine's portability. | |||
<center> | |||
[[Image:Heating_Block_Picture.png|300px|thumb|The heating block which will be re-sized]] </center> <br> | |||
'''Instructions'''<br> | '''Instructions'''<br> | ||
<br> | |||
'''Handles''' <br> | |||
The new panels with handles will be needed to be placed on specific sides of the machine. They will be placed on the wider sides of the machine across from one another. <br> | |||
'''Bigger Heating Block''' <br> | |||
Because the heating block has a new row and column it will now be 5 by 5 having nine more places for test tubes. This will require a larger lid and a larger heating block to be used during assembly to accommodate for this new bigger size. Besides those changes the over all assembly will remain the same. <br> | |||
'''New Materials For Casing''' <br> | |||
The aluminum side panels will be assembled in the same way the wooden panels are. This change will not require new assembly instructions. | |||
<!--- From Week 4 exercise ---> | <!--- From Week 4 exercise ---> | ||
| Line 60: | Line 68: | ||
'''Materials''' | '''Materials''' | ||
{| class="wikitable" | |||
|- | |||
! Supplied in PCR Kit !! Amount | |||
|- | |||
| PCR Machine || 1 | |||
|- | |||
| Fluorometer Box || 1 | |||
|- | |||
| Camera Stand || 1 | |||
|- | |||
| Glass Slides || 4 | |||
|- | |||
| Calf Thymus || 0.5 mL | |||
|- | |||
| Calibration DNA || 0.5 mL | |||
|- | |||
| GoTaq Polymerase and Buffer Solution || 10 mL | |||
|- | |||
| Small Test Tubes || 25 | |||
|- | |||
| Large Test Tubes || 25 | |||
|- | |||
| SYBR Green (diluted) || 1.5 mL | |||
|} | |||
{| class="wikitable" | |||
|- | |||
! Supplied by User !! Amount | |||
|- | |||
| Smartphone Camera || 1 | |||
|- | |||
| Laptop || 1 | |||
|- | |||
| ImageJ Download || 1 | |||
|- | |||
| Power Supply || 1 | |||
|- | |||
| DNA Sample || At least 3 samples | |||
|- | |||
|} | |||
'''PCR Protocol''' | '''PCR Protocol''' | ||
1. Using a micro-pipette, transfer 1.0-1.5 micro liters of desired DNA sample into at least three test tubes. | |||
2. Micro-pipette approximately 3.0 micro liters of reagent solution, including forward primer, reverse primer, GoTaq polymerase and buffer solution, into each of the test tubes with the DNA. | |||
3. Invert tubes, then turn upright to mix solution. | |||
4. Plug in OpenPCR Machine and turn it on. | |||
3. Open lid and place tubes into holder in PCR machine (can fit up to 25 tubes). Close the lid. | |||
4. Program the folowing cycles on the OpenPCR program: | |||
- Stage 1: 1 cycle, 95 degrees Celsius for 3 minutes | |||
- Stage 2: 30 cycles, 95 degrees for 30 seconds, 57 degrees for 30 seconds, 72 degrees for 30 second | |||
- Stage 3: 72 degrees for 3 minutes | |||
- Final Hold: 4 degrees | |||
5. Run reaction. | |||
6. After cycles are complete, open the lid and remove tubes for signal reading (see DNA Measurement Protocol below). | |||
'''DNA Measurement Protocol''' | '''DNA Measurement Protocol''' | ||
1. Remove lid from black box and invert the box, open the front flap. | |||
2. Place one glass slide into the holder. | |||
3. Depending on the amount of samples being used, take a clean pipette for each sample and label it so that it is only used for that sample of DNA. For example, label a pipette for SYBR green with a horizontal line on the pipette bulb, and label a pipette for diluted water with a vertical line on the bulb. Do NOT use pipettes for any other solution than what they are labelled for. | |||
4. Using the designated pipette, squeeze two drops of SYBR green dye inside the round glass windows on slide, preferably the second dot of the second row. | |||
5. Begin by adding a sample of diluted water to the SYBR green dye, and using the appropriate labelled pipette, add two drops in the same spot as the SYBR green dye. | |||
6. Align the sample so the blue LED shines directly though it focusing the light on the other side. | |||
7. Move holder apparatus inside the box so that no light reaches it. | |||
8. Place smartphone with the flash off onto holder and direct camera lens at the slide apparatus. | |||
9. Take photo with smartphone. | |||
10. E-mail the taken image to computer. | |||
11. On the computer open the image file using ImageJ (program can be downloaded from the Internet.) | |||
12. In ImageJ, under the "analyze" tool bar select "Set Measurements." | |||
13. Make sure the following boxes are checked, and no other boxes other than the ones listed: | |||
-Area | |||
-Mean Grey Value | |||
-Integrated Density | |||
14. From the download folder drag the image file into ImageJ. | |||
15. When the image is opened in ImageJ from the top drop down menu select "Image" and then "Color" and then "Split Channels." | |||
16. Close out the red and blue channel, only use the green channel | |||
17. With the Oval tool select only the entire drop of liquid, avoiding the background but encompassing every bit of the droplet. | |||
18. After the droplet part of the image is selected, go to "Analyze" then "Measure" or press Ctrl + M. | |||
19. Now move the circle to the background of the image by clicking and dragging on the circle, be careful not to change its size. | |||
20. Save the results as an excel spreadsheet (set by default). | |||
21. Once the Image has been processed, using a clean pipette, remove the sample of diluted water and SYBR green dye from the slide. | |||
22. Repeat steps 4-21 using included calf thymus DNA as a control, and move back on the glass slide two rows to eliminate contamination. | |||
23. Once the control has been analyzed, repeat step 4-21 using the sample DNA, ensuring to move back two rows on the glass slide each time a new sample is tested. | |||
24. Once there are no more rows on the glass slide, carefully discard the used slide and begin using a new one. | |||
==Research and Development== | ==Research and Development== | ||
| Line 82: | Line 200: | ||
<!--- A description of the diseases and their associated SNP's (include the database reference number and web link) ---> | <!--- A description of the diseases and their associated SNP's (include the database reference number and web link) ---> | ||
The SNP chosen for the lab is linked with Alzheimer's disease. Alzheimer's disease is the most common form of dementia. It has no cure and worsens as it progresses. Dementia is a loss of brain function and Alzheimer's disease affects memory, thinking, and behavior. The chosen SNP that is associated with an increase in risk for Alzheimer's disease is rs1050283, which is located on the 12 chromosome. More information is available from the NCBI website: | |||
http://www.ncbi.nlm.nih.gov/snp?term=rs1050283 | |||
| Line 89: | Line 208: | ||
<!--- Include the sequences of your forward and reverse primers. Explain why a disease allele will give a PCR product and the non-disease allele will not. ---> | <!--- Include the sequences of your forward and reverse primers. Explain why a disease allele will give a PCR product and the non-disease allele will not. ---> | ||
The sequence that is connected with an increase risk of Alzheimer's disease is CTGGGCOCGGACATGGAGGACGTG[C/T]GCGGCCGCCTGGTGCAGTACCGCG. An increase in the risk for Alzheimer's disease comes from the base change of C to T. | |||
The reverse primer would be GGAGGACGTG[T]GCGGCCGCCT and the forward primer would be CCTCCTGCAG[A]CGCCGGCGGA. | |||
A diseased allele will produce a PCR product because the gene associated with an increase risk that is trying to be tested for will have a T base in place of the normal C base. If the gene has this change in bases then the primers will be able to attach to the strands of DNA because the primers are designed to only attach to that specific sequence. | |||
| Line 96: | Line 217: | ||
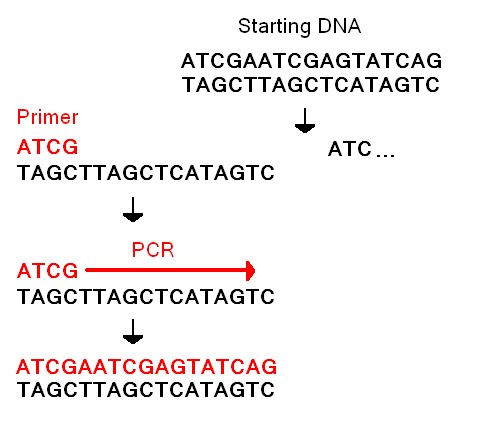
<!--- Include an illustration that shows how your system's primers allow specific amplification of the disease-related SNP ---> | <!--- Include an illustration that shows how your system's primers allow specific amplification of the disease-related SNP ---> | ||
[[Image:Primer.jpg]] | |||
Bayesian Stats | |||
[[Image:Prob-conditional-formula2.gif|200px|]] | |||
P(A|B) is the probability that Alzheimer’s disease will occur given a positive PCR test result (a T present instead of a C in the SNP), P(B|A) is the probability that a Alzheimer’s patient will test positive for the disease, P(A) is the probability of having the Alzheimer’s mutation, and P(B) is the probability of people without the Alzheimer’s disease mutation that yield positive results. | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
|} | |} | ||
Latest revision as of 14:05, 29 November 2012
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||||||||||||||||||||
OUR TEAMLAB 2 WRITE-UPThermal Cycler Engineering System Design  Key Features To begin with we will change the frame from a wooden construction to recycled aluminum. This will maintain the light-weight portability of the machine, but will greatly reduce the fire hazard of heating a wooden box to the required temperatures. Also, by eliminating the need for wood, the machine will be more eco-friendly. We will then add an additional row and column to the heating block to increase the product output without too much change to the overall size or cost of the machine. However, the increased productivity of the additional row and column will require the addition of a new larger heating lid. We will also add two carrying handles to either side of the machine. This will aid in the transport and increase the machine's portability.  Instructions
ProtocolsMaterials
1. Using a micro-pipette, transfer 1.0-1.5 micro liters of desired DNA sample into at least three test tubes. 2. Micro-pipette approximately 3.0 micro liters of reagent solution, including forward primer, reverse primer, GoTaq polymerase and buffer solution, into each of the test tubes with the DNA. 3. Invert tubes, then turn upright to mix solution. 4. Plug in OpenPCR Machine and turn it on. 3. Open lid and place tubes into holder in PCR machine (can fit up to 25 tubes). Close the lid. 4. Program the folowing cycles on the OpenPCR program: - Stage 1: 1 cycle, 95 degrees Celsius for 3 minutes - Stage 2: 30 cycles, 95 degrees for 30 seconds, 57 degrees for 30 seconds, 72 degrees for 30 second - Stage 3: 72 degrees for 3 minutes - Final Hold: 4 degrees 5. Run reaction. 6. After cycles are complete, open the lid and remove tubes for signal reading (see DNA Measurement Protocol below). DNA Measurement Protocol 1. Remove lid from black box and invert the box, open the front flap. 2. Place one glass slide into the holder. 3. Depending on the amount of samples being used, take a clean pipette for each sample and label it so that it is only used for that sample of DNA. For example, label a pipette for SYBR green with a horizontal line on the pipette bulb, and label a pipette for diluted water with a vertical line on the bulb. Do NOT use pipettes for any other solution than what they are labelled for. 4. Using the designated pipette, squeeze two drops of SYBR green dye inside the round glass windows on slide, preferably the second dot of the second row. 5. Begin by adding a sample of diluted water to the SYBR green dye, and using the appropriate labelled pipette, add two drops in the same spot as the SYBR green dye. 6. Align the sample so the blue LED shines directly though it focusing the light on the other side. 7. Move holder apparatus inside the box so that no light reaches it. 8. Place smartphone with the flash off onto holder and direct camera lens at the slide apparatus. 9. Take photo with smartphone. 10. E-mail the taken image to computer. 11. On the computer open the image file using ImageJ (program can be downloaded from the Internet.) 12. In ImageJ, under the "analyze" tool bar select "Set Measurements." 13. Make sure the following boxes are checked, and no other boxes other than the ones listed: -Area -Mean Grey Value -Integrated Density 14. From the download folder drag the image file into ImageJ. 15. When the image is opened in ImageJ from the top drop down menu select "Image" and then "Color" and then "Split Channels." 16. Close out the red and blue channel, only use the green channel 17. With the Oval tool select only the entire drop of liquid, avoiding the background but encompassing every bit of the droplet. 18. After the droplet part of the image is selected, go to "Analyze" then "Measure" or press Ctrl + M. 19. Now move the circle to the background of the image by clicking and dragging on the circle, be careful not to change its size. 20. Save the results as an excel spreadsheet (set by default). 21. Once the Image has been processed, using a clean pipette, remove the sample of diluted water and SYBR green dye from the slide. 22. Repeat steps 4-21 using included calf thymus DNA as a control, and move back on the glass slide two rows to eliminate contamination. 23. Once the control has been analyzed, repeat step 4-21 using the sample DNA, ensuring to move back two rows on the glass slide each time a new sample is tested. 24. Once there are no more rows on the glass slide, carefully discard the used slide and begin using a new one. Research and DevelopmentBackground on Disease Markers
Bayesian Stats P(A|B) is the probability that Alzheimer’s disease will occur given a positive PCR test result (a T present instead of a C in the SNP), P(B|A) is the probability that a Alzheimer’s patient will test positive for the disease, P(A) is the probability of having the Alzheimer’s mutation, and P(B) is the probability of people without the Alzheimer’s disease mutation that yield positive results. | |||||||||||||||||||||||||||||||||||