BME103:T930 Group 7 l2: Difference between revisions
No edit summary |
|||
| (14 intermediate revisions by 5 users not shown) | |||
| Line 14: | Line 14: | ||
|- | |- | ||
| [[Image:BME103student.jpg|100px|thumb|Name: Student: Elyse Candell<br>Role(s: Machine Engineer)]] | | [[Image:BME103student.jpg|100px|thumb|Name: Student: Elyse Candell<br>Role(s: Machine Engineer)]] | ||
| [[Image:BME103student.jpg|100px|thumb| | | [[Image:BME103student.jpg|100px|thumb|Wesley Karlin<br>Experimental Protocol Planner]] | ||
| [[Image:BME103student.jpg|100px|thumb|Name: Student<br>Role(s)]] | | [[Image:BME103student.jpg|100px|thumb|Name: Student<br>Role(s)]] | ||
| [[Image:BME103student.jpg|100px|thumb|Name: Student<br>Role(s)]] | | [[Image:BME103student.jpg|100px|thumb|Name: Student<br>Role(s)]] | ||
| Line 29: | Line 29: | ||
'''System Design'''<br> | '''System Design'''<br> | ||
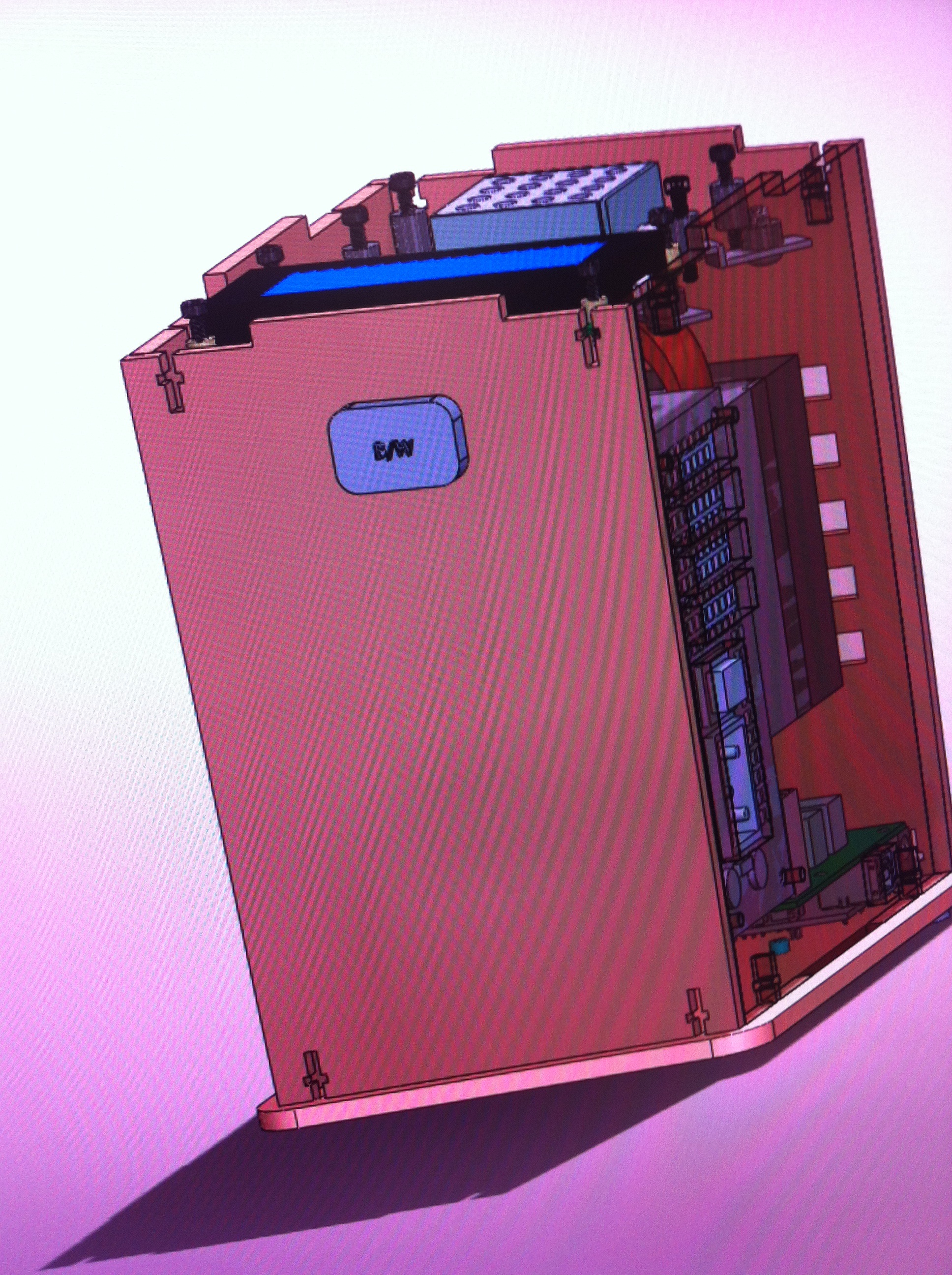
[[Image:systemchange.png]] | |||
The B/W part is a bluetooth/wifi sensor. This is to replace the computer. | |||
The outside of the Thermal Cycler is now made out of Polyurethane Foam Rigid instead of wood. | |||
'''Key Features'''<br> | '''Key Features'''<br> | ||
To make things easier, a smart phone will be able to control the the Thermal Cycler, like the computer did in our experiment. This is a button that will be pressed whenever a person wants to connect the Thermal Cycler either using their network or their bluetooth. | |||
The Polyurethane Foam Rigid is a better material to help keep heat in when the Thermal Cycler is heating up and keep it cool when the Thermal Cycler is cooling down. This helps it to heat up faster and cool down faster, thus promoting faster cycles. | |||
'''Instructions'''<br> | |||
1. Replace Wood panels with Polyurethane panels insulated with foam. | |||
2. Attach B/W sensor to the side of the OpenPCR machine and press to activate the sensor. | |||
3. Download the Open PCR Wireless App from the App Store onto your iPhone or smart phone. | |||
4. Run the app on your phone and sync it with the Open PCR machine. | |||
5. You are now ready to start using the machine. | |||
6. Type in the temperatures, times and press start. | |||
7. Run the process. | |||
8. Analyze your data. | |||
<!--- From Week 4 exercise ---> | <!--- From Week 4 exercise ---> | ||
| Line 96: | Line 114: | ||
'''PCR Protocol''' | '''PCR Protocol''' | ||
Sample: The kit is provided with the PCR reaction mix that contains all the ingredients to amplify DNA, except for the DNA wanted to be replicated. The reaction mix will come in bulk of 2 mL and the user should place 50 uL of the PCR reaction mix into one of the provided test tubes. Subsequently 50 uL of the DNA sample, provided by the user, should be inserted into the test tube containing the reaction mix. This concludes the steps needed to create the sample ready for DNA amplification as this tube will be inserted into the OpenPCR Machine. | |||
OpenPCR Machine: The OpenPCR Machine is provided in the kit and is already assembled for use and it just needs to be turned on. When turning it on there are two options for programming, and eventually receiving the results on, the machine. This can be accomplished by pushing the Bluetooth button on the machine or inserting a USB cord into the USB slot on the machine. If using Bluetooth, the Bluetooth on the user's phone also needs to be turned on and the programming options will open and can be set via smartphone; the same goes for a computer connected via USB cord. The machine must be programmed for five separate steps including the initial, the three various cycles, and the final. Each of these components have a box for inserting the degrees celsius required and the time slot allotted. The initial should be programmed at 95 degress celsius for 1 minute; the first of the cycles is 95 degrees celsius at 10 seconds, the second cycle is 57 degrees celsius at 10 seconds, and the third cycle is 72 degrees celsius at 10 seconds; the final should be 72 degrees celsius at 1 minute. These new, faster, and more efficient times and temperatures are courtesy of personal communication with Dr. Haynes. Once the programming is set, insert the testing tubes into the PCR machine and push start. Displayed on either the phone or computer should be an estimated time duration for how long it'll take to run the DNA amplification. When it's been completed the results will show up on either the smartphone or computer and the experiment will be completed! | |||
'''DNA Measurement Protocol'''<br> | |||
1. To keep the patient samples and solutions separate, 10 pipettes were labeled at the top with a permament marker with their corresponding sample<br> | |||
2. Take 10 Eppendorf tubes containing 400mL of buffer and label them with the permament marker to their corresponding sample<br> | |||
3. Next, transfer the patient sample into the assigned Eppendorf tube with an appropriate pipette. For example, Patient Sample A will be transferred into Eppendorf Tube A while using Pipette A only. This prevents the samples from contaminating each other.<br> | |||
4. Using the SYBR Green and its assigned pipette, two drops are placed in the first two center circles on the glass slide with the rough, superhydrophobic, Teflon layer<br> | |||
5. Then two drops of a patient sample were placed on top of the SYBR Green drops on the slide<br> | |||
6. With the SYBR Green mixed with the patient sample on the slide, the slide was positioned so that the blue ray of light would pass through the SYBR Green and patient sample<br> | |||
7. Afterwards, the smarphone operator took a picture of the slide under the following conditions: no light present in the area, an inactive flash, iso changed to 800, white balance set to auto, exposure set to maximum, and the contrast is set to minimum.<br> | |||
8. The picture was then sent to the Image J software for processing.<br> | |||
9. This process was repeated for all 6 patient samples, a positive and negative control, Calf Thymus DNA, and pure water.<br> | |||
''' | '''ImageJ Software Protocol'''<br> | ||
1. Email the photos to an email address, open the photos on a computer, and save them to the computer<br> | |||
2. Open ImageJ and select a photo<br> | |||
3. Once the photo is opened in ImageJ, click on Analyze -> Set Measurements -> Area Integrated Density and Mean Grey Value<br> | |||
4. Go to Image, select Color, and then choose the option Split Screen<br> | |||
5. Using the green channel only and the oval tool, draw an oval around the drops of Patient Sample and SYBR Green on the slide<br> | |||
6. Next, go to Analyze -> Measure -> Save Results as an Excel file<br> | |||
7. To collect background measurements, draw a new oval but in the background and follow step 6<br> | |||
8. Complete this process for all the photos taken<br><br> | |||
==Research and Development== | ==Research and Development== | ||
| Line 110: | Line 150: | ||
<!--- A description of the diseases and their associated SNP's (include the database reference number and web link) ---> | <!--- A description of the diseases and their associated SNP's (include the database reference number and web link) ---> | ||
rs74315509 <br> | SNP: rs74315509 <br> | ||
This SNP is linked to schizophrenia. <br> | CGC changed to CAC <br> | ||
This SNP is linked to schizophrenia. Schizophrenia is a mental disorder with numerous symptoms, including hallucinations, delusions, and abnormal social behavior. It is usually triggered by extreme anxiety in people who are genetically predisposed. | |||
<br> | |||
http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=74315509 | http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=74315509 | ||
Latest revision as of 00:26, 29 November 2012
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||
OUR TEAM
LAB 2 WRITE-UPThermal Cycler EngineeringOur re-design is based upon the Open PCR system originally designed by Josh Perfetto and Tito Jankowski. The B/W part is a bluetooth/wifi sensor. This is to replace the computer. The outside of the Thermal Cycler is now made out of Polyurethane Foam Rigid instead of wood.
The Polyurethane Foam Rigid is a better material to help keep heat in when the Thermal Cycler is heating up and keep it cool when the Thermal Cycler is cooling down. This helps it to heat up faster and cool down faster, thus promoting faster cycles. Instructions 2. Attach B/W sensor to the side of the OpenPCR machine and press to activate the sensor. 3. Download the Open PCR Wireless App from the App Store onto your iPhone or smart phone. 4. Run the app on your phone and sync it with the Open PCR machine. 5. You are now ready to start using the machine. 6. Type in the temperatures, times and press start. 7. Run the process. 8. Analyze your data.
ProtocolsMaterials
PCR Protocol Sample: The kit is provided with the PCR reaction mix that contains all the ingredients to amplify DNA, except for the DNA wanted to be replicated. The reaction mix will come in bulk of 2 mL and the user should place 50 uL of the PCR reaction mix into one of the provided test tubes. Subsequently 50 uL of the DNA sample, provided by the user, should be inserted into the test tube containing the reaction mix. This concludes the steps needed to create the sample ready for DNA amplification as this tube will be inserted into the OpenPCR Machine. OpenPCR Machine: The OpenPCR Machine is provided in the kit and is already assembled for use and it just needs to be turned on. When turning it on there are two options for programming, and eventually receiving the results on, the machine. This can be accomplished by pushing the Bluetooth button on the machine or inserting a USB cord into the USB slot on the machine. If using Bluetooth, the Bluetooth on the user's phone also needs to be turned on and the programming options will open and can be set via smartphone; the same goes for a computer connected via USB cord. The machine must be programmed for five separate steps including the initial, the three various cycles, and the final. Each of these components have a box for inserting the degrees celsius required and the time slot allotted. The initial should be programmed at 95 degress celsius for 1 minute; the first of the cycles is 95 degrees celsius at 10 seconds, the second cycle is 57 degrees celsius at 10 seconds, and the third cycle is 72 degrees celsius at 10 seconds; the final should be 72 degrees celsius at 1 minute. These new, faster, and more efficient times and temperatures are courtesy of personal communication with Dr. Haynes. Once the programming is set, insert the testing tubes into the PCR machine and push start. Displayed on either the phone or computer should be an estimated time duration for how long it'll take to run the DNA amplification. When it's been completed the results will show up on either the smartphone or computer and the experiment will be completed!
Research and DevelopmentBackground on Disease Markers SNP: rs74315509
| ||||||||||||||||||||||||||||||||||