BME103:T930 Group 9 l2: Difference between revisions
Devraj Patel (talk | contribs) |
|||
| (31 intermediate revisions by 4 users not shown) | |||
| Line 12: | Line 12: | ||
{| style="wikitable" width="700px" | {| style="wikitable" width="700px" | ||
|- | |- valign="top" | ||
| [[Image:Jasmine.jpg|100px|thumb|Devraj Patel<br>Open PCR Machine Engineer ]] | | [[Image:Jasmine.jpg|100px|thumb|Devraj Patel<br>Open PCR Machine Engineer ]] | ||
| [[Image:Snowwhite.jpg|100px|thumb|Brandon Simmons<br>Open PCR Machine Engineer ]] | | [[Image:Snowwhite.jpg|100px|thumb|Brandon Simmons<br>Open PCR Machine Engineer ]] | ||
| Line 27: | Line 27: | ||
Our re-design is based upon the [http://openpcr.org Open PCR] system originally designed by Josh Perfetto and Tito Jankowski.<br> | Our re-design is based upon the [http://openpcr.org Open PCR] system originally designed by Josh Perfetto and Tito Jankowski.<br> | ||
'''System Design:'''<br> | |||
'''''Open PCR Gen. 2'''<br>'' | |||
[[Image:BRANDON'S lab 2.png]] | [[Image:BRANDON'S lab 2.png]] | ||
'''Key Features'''<br> | '''Key Features'''<br> | ||
We introduced two new | <br> | ||
We introduced two new design features to create a machine that would allow the generation 2 PCR machine to be more portable and reduce the need for external equipment besides the machine itself. | |||
The first of the two features is a better performing LCD screen with attached four analog buttons on the bottom of screen that allow for the programmer to program the machine and eliminate the need of a computer to program. The four analog buttons will include: Increase, Decrease, Temp/Time, Start/Stop/Restart. These buttons will allow the programmer to prepare a PCR experiment sequence without the use of external equipment, such as a computer equipped with PCR software. These buttons will be placed directly below the LCD screen, so that they can be easily accessible and usable. | The first of the two new features is a better performing LCD screen with attached four analog buttons on the bottom of screen that allow for the programmer to program the machine and eliminate the need of a computer to program. The four analog buttons will include: Increase, Decrease, Temp/Time, Start/Stop/Restart. These buttons will allow the programmer to prepare a PCR experiment sequence without the use of external equipment, such as a computer equipped with PCR software. These buttons will be placed directly below the LCD screen, so that they can be easily accessible and usable. | ||
The second of the two features includes a new internal PCR Board, which is configured with the new LCD screen. Since the programmer will now be able to change the program on the machine itself, the internal PCR Board will have to be modified to allow the program to be stored and set in the PCR Board. The changes that need to be taken with this equipment are to wire the buttons from the LCD screen to the PCR Board, so that the inputs by the user can be recognized by the system and the PCR board will also need a new program implemented into the system that can understand the user inputs as well. With these small design changes, we will eliminate the reliance on other technologies to operate the machine and increase its overall mobility. | The second of the two new features includes a new internal PCR Board, which is configured with the new LCD screen. Since the programmer will now be able to change the program on the machine itself, the internal PCR Board will have to be modified to allow the program to be stored and set in the PCR Board. The changes that need to be taken with this equipment are to wire the buttons from the LCD screen to the PCR Board, so that the inputs by the user can be recognized by the system and the PCR board will also need a new program implemented into the system that can understand the user inputs as well. With these small design changes, we will eliminate the reliance on other technologies to operate the machine and increase its overall mobility. With these small changes it will allow the machine to be used around the world. | ||
<br> | |||
'''Instructions'''<br> | '''Instructions'''<br> | ||
<br> | |||
To add these design changes, first, the previous LCD screen would need to be removed and new space must be cut out for the larger screen with attached buttons, underneath the LCD screen space that is already there. Second, the wiring for the new screen must be taken care of for the user's inputs to be read. So, we must attach the new screen to the previous cable attaching the previous LCD screen and create wiring that connects the four buttons to the new PCR board. Thirdly, we must take out the PCR board and install the modified PCR board using previous mounting spots, allowing for the program to be set and stored in the internal memory of the new PCR board. Remembering to install the new wiring with both the new LCD screen and the analog buttons, we can reassemble machine and test for operating efficiency. <br> | To add these design changes, first, the previous LCD screen would need to be removed and new space must be cut out for the larger screen with attached buttons, underneath the LCD screen space that is already there. Second, the wiring for the new screen must be taken care of for the user's inputs to be read. So, we must attach the new screen to the previous cable attaching the previous LCD screen and create wiring that connects the four buttons to the new PCR board. Thirdly, we must take out the PCR board and install the modified PCR board using previous mounting spots, allowing for the program to be set and stored in the internal memory of the new PCR board. Remembering to install the new wiring with both the new LCD screen and the analog buttons, we can reassemble machine and test for operating efficiency. <br> | ||
<!--- From Week 4 exercise ---> | <!--- From Week 4 exercise ---> | ||
<br><br> | <br><br> | ||
| Line 66: | Line 68: | ||
<!--- Place your two tables "Supplied in the kit" and "Supplied by User" here ---> | <!--- Place your two tables "Supplied in the kit" and "Supplied by User" here ---> | ||
'''''Supplied in the kit''''' | |||
{|border="1" cellpadding="5" | |||
|- | |||
! scope="col" | Material | |||
! scope="col" | Amount | |||
|- | |||
|Open PCR Machine | |||
|1 | |||
|- | |||
|Mircopipette | |||
|1 | |||
|- | |||
|Sets of PCR Tubes (containing 6 individual tubes) | |||
|8 (replaced as needed by user) | |||
|- | |||
|Reagent'''*''' | |||
|1000μL | |||
|- | |||
|Electrical cord | |||
|1 | |||
|- | |||
|Fluorimeter | |||
|1 | |||
|- | |||
|Glass slides | |||
|supplied as needed | |||
|- | |||
|Black box | |||
|1 | |||
|- | |||
|Phone holder | |||
|1 | |||
|} | |||
<br> | |||
{|border="1" cellpadding="5" | |||
|- | |||
! scope="col" | Reagent'''*''' | |||
! scope="col" | Volume | |||
|- | |||
|Template DNA (20 ng) | |||
|0.2 μL | |||
|- | |||
|10 μM Forward Primer | |||
|1.0μL | |||
|- | |||
|10 μM Reverse Primer | |||
|1.0μL | |||
|- | |||
|GoTaq master mix | |||
|50μL | |||
|- | |||
|dH2O | |||
|47.8 μL | |||
|- | |||
|Total Volume | |||
|100.0 μL | |||
|} | |||
<br> | |||
'''''Supplied by user''''' | |||
{|border="1" cellpadding="5" | |||
|- | |||
! scope="col" | Material | |||
! scope="col" | Amount | |||
|- | |||
|DNA samples | |||
|supplied as needed | |||
|- | |||
|USB | |||
|1 | |||
|- | |||
|Assembly tools | |||
|1 set | |||
|- | |||
|Smartphone | |||
|1 | |||
|} | |||
<br><br> | |||
'''PCR Protocol''' <br> | |||
Steps to Run PCR<br> | |||
#Connect the PCR machine to the computer.<br> | |||
#Open the 'OpenPCR' program on the computer.<br> | |||
#Label the tubes. This information should include the patient number (1 or 2) or control (+ or -), as well as the replication number (1, 2, or 3).<br> | |||
#Prepare the experiment by inserting the reactants into the PCR tubes. These tubes will consist of the patients DNA, along with the other provided mixing components'''*'''. After filling each tube, put it into the chamber at the top of the machine.<br> | |||
#Close and tighten the lid of the chamber.<br> | |||
#Customize the settings in the 'Thermal Cycler' program to include three stages: Stage 1 - one cycle that will heat the reactants up to 95 degrees Celsius for three minutes, Stage 2 - 35 cycles that will heat the reactants to 95 degrees Celsius for 30 seconds, 57 degrees Celsius for 30 seconds, and 72 degrees Celsius for 30 seconds. <br> | |||
#Press start on the program to begin running the PCR.<br> | |||
#Collect and record data at the completion of the trial.<br> | |||
<br> | |||
'''DNA Measurement Protocol'''<br> | |||
Fluorimeter Assembly <br> | |||
#Place a glass slide on the device. <br> | |||
#Using a pipette, add two drops of water to the slide. <br> | |||
#Turn on the blue LED light, and adjust the slide so that the light shines directly through the center of the water drop. <br> | |||
#Adjust the camera settings on a smartphone as follows: <br> | |||
#*Turn off the flash <br> | |||
#*Set exposure to the highest setting <br> | |||
#*Set saturation to the highest setting <br> | |||
#*Set contrast to the lowest setting <br> | |||
#Place the smartphone on the phone holder and position it in front the of fluorimeter device. Make sure to place in same location for each trial to avoid errors. <br> | |||
#Cover the entire setup with a black box in order to create as dark of an environment as possible. <br> | |||
#Take a picture with the smartphone. For best results, set the camera timer on the smartphone in order to be able to take a picture with the box completely closed. <br> <br> | |||
How to Open Pictures Using Image J <br> | |||
#Using the smartphone, email the images to someone in the group with a computer. <br> | |||
#From the computer, open the email and download the images. <br> | |||
#Save the images to the computer. <br> | |||
#If the computer does not already have Image J installed, the program can be downloaded by going to http://rsb.info.nih.gov/ij/download.html . <br> | |||
#In Image J, go to file, open, and then select the desired picture. <br><br> | |||
How to Analyze Pictures in Image J<br> | |||
#With the desired picture open, go to analyze, set measurements, and select Area Integrated Density and Mean Grey Value.<br> | |||
#Go to image, color, and then split screen.<br> | |||
#In the green channel, use the oval tool to draw an oval around the water drop.<br> | |||
#Go to analyze, measure, and save the results as an Excel file.<br> | |||
#To get background measurements, draw a similar-sized oval somewhere in the background. Then, go to analyze, measure, and save the results as an Excel file.<br><br> | |||
==Research and Development== | |||
| Line 121: | Line 231: | ||
2.) Forward primer:5" TGCTGGTTTTAGCACTGACA 3" (at position 5009175) | 2.) Forward primer:5" TGCTGGTTTTAGCACTGACA 3" (at position 5009175) | ||
<br> Reverse primer:5" GCACTCTTC[ | <br> Reverse primer:5" GCACTCTTC[G]CATGGAGTTG 3" | ||
These primers are designed to be 150 bp apart in order to run the PCR faster than normal, 200 bp, at rate of 10 seconds per cycle. | |||
The disease allele will give a positive result because the primers, forward and revers, are designed specifically to attach only to DNA strand in PCR. On the other hand, a non-disease allele will give a negative result due to the fact that the primers will not attach to the DNA strand in PCR. | |||
| Line 128: | Line 244: | ||
<!--- Include an illustration that shows how your system's primers allow specific amplification of the disease-related SNP ---> | <!--- Include an illustration that shows how your system's primers allow specific amplification of the disease-related SNP ---> | ||
[[Image:Jej.png]] | |||
[[Image:BME103_2012_classpic2.png]] | [[Image:BME103_2012_classpic2.png]] | ||
[[Image: | |||
<!--- Bonus: explain how Bayesian statistics can be used to assess the reliability of your team's method. Just write the equation using variables that are relevant to your team's new test. You do not need actual numbers ---> | |||
== Bayes’ theorem == | |||
[[Image:Bayes-rule.png]] | |||
P(A):is the probabilities of having a disease <br> | |||
P(B): is the probabilities of non-disease patients who have a positive result<br> | |||
P(A/B): is the probabilities of having a positive result in PCR <br> | |||
P(B/A): is the probabilities of a patients with a disease who have a positive result <br> | |||
Latest revision as of 18:44, 29 November 2012
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||||||||||||||||||||||||||||||
OUR TEAMLAB 2 WRITE-UPThermal Cycler EngineeringOur re-design is based upon the Open PCR system originally designed by Josh Perfetto and Tito Jankowski. System Design: Open PCR Gen. 2
The first of the two new features is a better performing LCD screen with attached four analog buttons on the bottom of screen that allow for the programmer to program the machine and eliminate the need of a computer to program. The four analog buttons will include: Increase, Decrease, Temp/Time, Start/Stop/Restart. These buttons will allow the programmer to prepare a PCR experiment sequence without the use of external equipment, such as a computer equipped with PCR software. These buttons will be placed directly below the LCD screen, so that they can be easily accessible and usable. The second of the two new features includes a new internal PCR Board, which is configured with the new LCD screen. Since the programmer will now be able to change the program on the machine itself, the internal PCR Board will have to be modified to allow the program to be stored and set in the PCR Board. The changes that need to be taken with this equipment are to wire the buttons from the LCD screen to the PCR Board, so that the inputs by the user can be recognized by the system and the PCR board will also need a new program implemented into the system that can understand the user inputs as well. With these small design changes, we will eliminate the reliance on other technologies to operate the machine and increase its overall mobility. With these small changes it will allow the machine to be used around the world.
ProtocolsMaterials
Supplied by user
PCR Protocol
DNA Measurement Protocol
How to Open Pictures Using Image J
How to Analyze Pictures in Image J
Research and DevelopmentBackground on Disease Markers
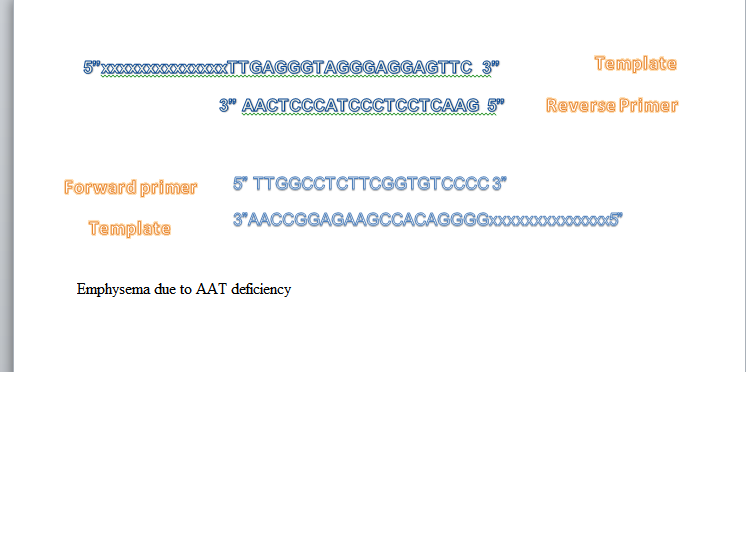
The marker that is being used is rs709932[2].This SNP is associated with Emphysema due to AAT deficiency. The sequence associated with Emphysema due to AAT deficiency is C(A)T , while a normal sequence is C(G)T, which is located on the 14 chromosome. The gene alteration leads to a mutated human protein. It goes from R[Arg] to H[His].
Emphysema due to AAT deficiency
The marker that is being used is rs137852466 [4]. This SNP is associated with Hemophilia A. The sequence associated with Hemophilia A is C(G)C , while a normal sequence is C(A)C, which is located on the X chromosome.The gene alteration leads to a mutated human protein. It goes from R[Arg] to H[His].
Primer Design
These primers are designed to be 150 bp apart in order to run the PCR faster than normal, 200 bp, at rate of 10 seconds per cycle. The disease allele will give a positive result because the primers, forward and revers, are designed specifically to attach only to DNA strand in PCR. On the other hand, a non-disease allele will give a negative result due to the fact that the primers will not attach to the DNA strand in PCR.
Bayes’ theoremP(A):is the probabilities of having a disease
| |||||||||||||||||||||||||||||||||||||||||||||