BME103 s2013:T900 Group2 L3: Difference between revisions
Shang Ruan (talk | contribs) |
|||
| (19 intermediate revisions by 5 users not shown) | |||
| Line 15: | Line 15: | ||
| [[Image:BME103student.jpg|100px|thumb|Name: Joe Sansone<br>Role(R&D Scientist)]] | | [[Image:BME103student.jpg|100px|thumb|Name: Joe Sansone<br>Role(R&D Scientist)]] | ||
| [[Image:photo(3).jpg|100px|thumb|Name: Shang Ruan <br> Open PCR Machine Engineer]] | | [[Image:photo(3).jpg|100px|thumb|Name: Shang Ruan <br> Open PCR Machine Engineer]] | ||
| [[Image:BME103student.jpg|100px|thumb|Name: | | [[Image:BME103student.jpg|100px|thumb|Name: William Scott<br>Role(R&D Scientist)]] | ||
| [[Image: | | [[Image:Funny-monkey-05.jpg|100px|thumb|Name: Andy Son<br>Protocol Planner]] | ||
| [[Image: | | [[Image:404005_4622115081677_1186647743_n.jpg|100px|thumb|Mitch Riggs <br> Open PCR Machine Engineer/ Team Leader]] | ||
|} | |} | ||
| Line 30: | Line 30: | ||
| Sample Name || Ave. INTDEN* || Calculated μg/mL || Conclusion (pos/neg) | | Sample Name || Ave. INTDEN* || Calculated μg/mL || Conclusion (pos/neg) | ||
|- | |- | ||
| Positive Control || | | Positive Control || 518016 || 0.199 || N/A | ||
|- | |- | ||
| Negative Control || | | Negative Control || 375033 || 0.032 || N/A | ||
|- | |- | ||
| Tube Label: | | Tube Label:2 Patient ID: 91562 rep 1 || 384551 || 0.043 || neg | ||
|- | |- | ||
| Tube Label: | | Tube Label:3 Patient ID: 91562 rep 2 || 312171 || -0.042 || neg | ||
|- | |- | ||
| Tube Label: | | Tube Label:4 Patient ID: 91562 rep 3 || 371384 || -0.031 || neg | ||
|- | |- | ||
| Tube Label: | | Tube Label:5 Patient ID: 25235 rep 1 || 467127 || 0.139 || pos | ||
|- | |- | ||
| Tube Label: | | Tube Label:6 Patient ID: 25235 rep 2 || 406664 || 0.064 || neg | ||
|- | |- | ||
| Tube Label: | | Tube Label:7 Patient ID: 25235 rep 3 || 484088 || 0.154 || pos | ||
|} | |} | ||
<nowiki>* Ave. INTDEN = | <nowiki>* Ave. INTDEN (Patient 91562)= 356035.3 </nowiki> | ||
<nowiki>*Ave. INTDEN (Patient 25235)= 452626.3 </nowiki> | |||
| Line 56: | Line 57: | ||
Calculation 1: The probability that the sample actually has the cancer DNA sequence, given a positive diagnostic signal.<br> | Calculation 1: The probability that the sample actually has the cancer DNA sequence, given a positive diagnostic signal.<br> | ||
* A = [ | * A = [frequency of cancer-positive conclusions] = [9/20] = [0.45] | ||
* B = [ | * B = [frequency of positive PCR reactions] = [26/60] = [0.43] | ||
* P (B|A) = [ | * P (B|A) = [frequency of positive PCR given cancer-positive conclusion] = [24/27] = [0.89] | ||
* '''P(A|B) = [ | * '''P(A|B) = [0.931 0r 93.1%]''' | ||
<br> | <br> | ||
| Line 71: | Line 72: | ||
Calculation 3: The probability that the patient will develop cancer, given a cancer DNA sequence.<br> | Calculation 3: The probability that the patient will develop cancer, given a cancer DNA sequence.<br> | ||
* A = [ | * A = [frequency of "yes" cancer diagnosis] = [6/9] = [0.67] | ||
* B = [ | * B = [frequency of pos test conclusions] = [9/20] = [0.45] | ||
* P (B|A) | * P (B|A) = [frequency of pos given "yes"] = [0.67] | ||
* '''P(A|B) = [ | * '''P(A|B) = [0.47 or 47%]''' | ||
<br> | <br> | ||
| Line 89: | Line 90: | ||
'''We concluded that a good system ''Must Have'':''' | '''We concluded that a good system ''Must Have'':''' | ||
* [Must have #1 - | * [Must have #1 - Be easy to determine the result. Normally we have bunches of tubes waiting to run the test. Efficiency is important for the test. If it takes sophisticated procedure to determine the result,the test engineer must be highly qualified which is not that common. ] | ||
* [Must have #2 - | * [Must have #2 - Fast imaging results. Efficiency is essential when the test we are running is based on tons of samples.] | ||
* [Must have #3& #4 - Simple OpenPCR software to operate and easy sample loading OpenPCR. Basically the same reason as #1. ] | |||
* [Must have #5 - Small sample volume. Operating the whole test is easier with small samples. We don't have to waste time on collecting big amount of sample and then disposing it. ] | |||
'''We concluded that we would ''Want'' a good system to have:''' | '''We concluded that we would ''Want'' a good system to have:''' | ||
* [Want #1 - | * [Want #1 - Low cost. Nobody wants to pay more than they have to.] | ||
* [Want #2 - | * [Want #2 - Portable and compact. A small portable machine makes use much simpler and hassle-free for use anywhere.] | ||
'''We concluded that a good system ''Must Not Have'':''' | '''We concluded that a good system ''Must Not Have'':''' | ||
* [Must Not Have #1 - | * [Must Not Have #1 - Bad USB connectivity. The whole experiment was dependent on transferring data via USB. If it doesn't work the whole system is compromised.] | ||
* [Must Not Have #2 - | * [Must Not Have #2 - Fire hazard. Dangerous equipment is worse than broken equipment. Safety is #1.] | ||
'''We concluded that a good system ''Should Avoid'':''' | '''We concluded that a good system ''Should Avoid'':''' | ||
* [Should Avoid #1 - | * [Should Avoid #1 - Inaccurate time readout. Accurately estimating time can increase productivity a lot.] | ||
* [Should Avoid #2 - | * [Should Avoid #2 - Slow amplification. Three hours for one batch of DNA is too long to wait in commercial applications.] | ||
| Line 125: | Line 129: | ||
<!-- If your team decided to change any of the machinery/ devices, summarize the new features here and delete the '''We chose keep the devices the same as the original system''' section. --> | <!-- If your team decided to change any of the machinery/ devices, summarize the new features here and delete the '''We chose keep the devices the same as the original system''' section. --> | ||
'''We chose to include these new features''' | '''We chose to include these new features''' | ||
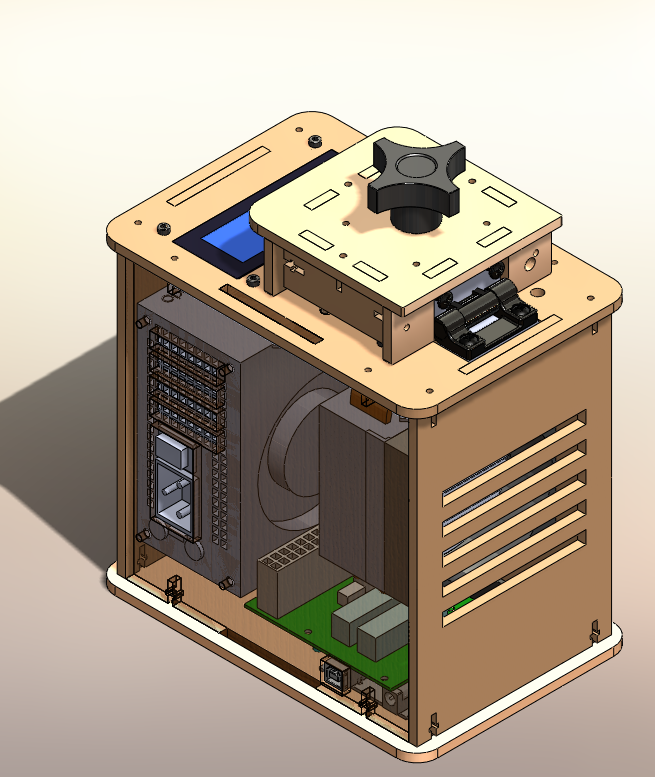
* Feature 1 - | * Feature 1 - We thought we would provide the PCR with an additional flourimeter apparatus (designed like a mini-box that a smart phone can be incorporated into) as well as the necessary chemicals. | ||
* Feature 2 - | * Feature 2 - We would provide an app for smart phone that the user buys. The app would in theory do all the necessary calculations in one easy step (pic->result), basically skipping the image j analysis. | ||
| Line 152: | Line 147: | ||
<!-- If your team decided to change the PCR and/or the Fluorimeter imaging protocols, summarize the new approaches/ features here and delete the '''We chose keep the protocols the same as the original system''' section. --> | <!-- If your team decided to change the PCR and/or the Fluorimeter imaging protocols, summarize the new approaches/ features here and delete the '''We chose keep the protocols the same as the original system''' section. --> | ||
'''We chose to include these new approaches/ features''' | '''We chose to include these new approaches/ features''' | ||
* | *We thought we would provide the PCR with an additional flourimeter apperatus (designed like a mini-box that a smart phone can be incorperated into) as well as the necessary chemicals. The user would have to provide some DNA sample (saliva or blood maybe) and we would provide an app for smart phone that the user buys. The app would in theory do all the necessary calculations in one easy step (pic->result), basically skipping the image j analysis. | ||
'''MATERIALS''' | '''MATERIALS''' | ||
<br> | |||
< | '''Supplied In Kit''' | ||
<br> | |||
dNTPs | |||
<br> | |||
MgCl2 | |||
<br> | |||
Reaction buffers | |||
<br> | |||
Taq DNA Polymerase | |||
<br> | |||
<br> | |||
'''Supplied by User''' | |||
<br> | |||
Sample DNA | |||
<br> | |||
Forward and Reverse Primers | |||
<br> | |||
Fluorescent, cancer-specific probe | |||
<br> | |||
SYBR Green dye | |||
| Line 174: | Line 178: | ||
* '''PCR Protocol''' | * '''PCR Protocol''' | ||
<!-- Create a step-by-step procedure for setting up and running PCR reactions. Your instructions should include everything from adding reagents to the tubes, to programming the PCR machine and running the reaction.--> | <!-- Create a step-by-step procedure for setting up and running PCR reactions. Your instructions should include everything from adding reagents to the tubes, to programming the PCR machine and running the reaction.--> | ||
*''' Step 1:''' The first step was to program the PCR machine and create the Thermal Cycler Program. The set-up used for the program is as follows | |||
'''Stage one:''' 1 cycle, 95 degrees Celsius for 3 minutes | |||
<br> | |||
'''Stage two:''' 35 cycles, 95 degrees Celsius for 30 seconds, 57 degrees Celsius for 30 seconds, 72 degrees Celsius for 30 seconds | |||
<br> | |||
'''Stage three:''' 72 degrees Celsius for 3 minutes | |||
<br> | |||
'''Final Hold:''' 4 degrees Celsius | |||
* '''Step 2:''' The second step is to add reagents to the tubes. Start by gathering the necessary materials to set-up the DNA samples (pipette, PCR reaction mix, 8 transfer pipettes) | |||
* '''Step 3:''' Set the pipette to 50 microliters | |||
*''' Step 4:''' Place the transfer pipette onto the pipette to prevent cross contamination (never re-use). | |||
* '''Step 5:''' Use the pipette to transfer 50 microliters of each tube in the PCR reaction mix and transfer accordingly to the DNA sample tubes corresponding to the labels. | |||
* '''Step 6:''' Place the set of mixed tubes into the Open PCR machine and shut it tightly. | |||
* '''Step 7:''' Hook up the machine to a computer and run the Open PCR application with the pre set-up Thermal Cycler program. | |||
* '''Step 8:''' Once the application finishes (approximately 1 hour and 30 minutes) the DNA sample has been set up. | |||
* '''DNA Measurement and Analysis Protocol''' | * '''DNA Measurement and Analysis Protocol''' | ||
<!-- Create a step-by-step procedure for measuring DNA amplification in the PCR reactions. Your instructions should include everything from diluting the samples in SYBR Green, to placing the drops onto the fluorimeter (if your group is using the fluorimeter), to collecting and processing images in Image J. Don't forget to provide instructions on how to set up the calf thymus DNA samples for calibration, and how to convert INTDEN values into concentrations.---> | <!-- Create a step-by-step procedure for measuring DNA amplification in the PCR reactions. Your instructions should include everything from diluting the samples in SYBR Green, to placing the drops onto the fluorimeter (if your group is using the fluorimeter), to collecting and processing images in Image J. Don't forget to provide instructions on how to set up the calf thymus DNA samples for calibration, and how to convert INTDEN values into concentrations.---> | ||
* '''Step 1:''' The first step was to set up a camera on a smart phone that can take a photo of the reactions mixed with the SYBR Green most accurately. The following is what settings our group had for the camera. | |||
<br> | |||
'''Smart Phone Camera Settings'''<br> | |||
* ''The smartphone used for the camera was the Android Google Nexus 4. An app named Camera Self-Timer was installed to create a window in which the camera can shoot a picture in which the fluorimeter could then be covered in darkness for the most accurate results.'' | |||
** Flash: No flash was used | |||
** ISO setting: Unknown (could not be altered) | |||
** White Balance: White balance was set on auto | |||
** Exposure: Exposure was set on auto | |||
** Saturation: Saturation was set on high | |||
** Contrast: Contrast was set on low | |||
* '''Step 2:''' The second step was to dilute the samples in SYBR Green. Each mixture was created by transferring the appropriate solution using a micropipette that was set to 80 μL. The mixtures were created corresponding to the following table... | |||
<br> | |||
'''Solutions Used for Calibration''' | |||
{| {{table}} width=700 | |||
|- | |||
| '''Calf Thymus DNA Solution (microg/mL)''' || '''Volume 2X DNA Solution (uL)''' || '''Volume SYBR GREEN I Solution (uL)'''|| '''Fina DNA concentration in PicoGreen Assay (ng/mL)''' | |||
|- | |||
| 5 || 80 || 80 || 2.5 | |||
|- | |||
| 2 || 80 || 80 || 1 | |||
|- | |||
| 1|| 80|| 80|| 0.5 | |||
|- | |||
| 0.5 || 80|| 80 || 0.25 | |||
|- | |||
| 0.25 || 80 || 80|| 0.125 | |||
|- | |||
| 0 || 80 || 80|| blank | |||
|} | |||
* '''Step 3:''' The solutions above were mixed by combining drops of 80 μL onto the slide on the flourimeter. It was then lined up with the blue LED light that emitted from the flourimeter. After each transfer of a solution onto the slide, a different beaker was used to prevent cross-contamination. | |||
* '''Step 4:''' The camera was placed on a cradle that was in approximately equal height of the fluorimeter. If the cradle and camera needed to be taller in order to be of equal height to the fluorimeter, the cradle was then placed on a stacked glass case until the camera was parallel. The distance from the cradle to the fluorimeter was about 7 cm. After creating a solution for calibration the camera was then set on a self-timer of 10 seconds and then the fluorimeter was encased in a box and covered for complete darkness for the most accurate results. After the beep indicating that the picture was taken the cycle was then complete and the process was then repeated for each solution. | |||
* '''Step 5:''' The photos are uploaded onto the image j application. Then do the command Split Channels, splitting the pic into 3 new ones. Choose the one labeled green. The measurement tool should be set to measure IntDen (or intergrated density). Using the oval tool, set the oval over majority of the droplet, avoiding reflections as best as possible. Hit the command Measure under Analyze, and a table should come up, giving IntDen value | |||
<br><br> | <br><br> | ||
| Line 216: | Line 263: | ||
'''Our primers address the following design needs''' | '''Our primers address the following design needs''' | ||
* | * The PCR method utilized above produces very effective results because the primer annealing follows base pairing rules therefore, by isolating the targeted SNP on the DNA template strand and amplifying them the cancerous genes can be detected easier. | ||
<br><br> | <br><br> | ||
Latest revision as of 08:23, 16 April 2013
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAMLAB 3 WRITE-UPOriginal System: PCR ResultsPCR Test Results
* Ave. INTDEN (Patient 91562)= 356035.3 *Ave. INTDEN (Patient 25235)= 452626.3
Bayes Theorem equation: P(A|B) = P(B|A) * P(A) / P(B)
Calculation 3: The probability that the patient will develop cancer, given a cancer DNA sequence.
New System: Design StrategyWe concluded that a good system Must Have:
We concluded that we would Want a good system to have:
[[Image:
KEY FEATURES We chose to include these new features
New System: ProtocolsDESIGN We chose to include these new approaches/ features
Stage one: 1 cycle, 95 degrees Celsius for 3 minutes
New System: Research and DevelopmentBACKGROUND Polymerase Chain Reaction (PCR) is a scientific method that utilizes DNA Polymerase to create a complimentary base strand from a template strand of DNA. Triphosphate nucleotides align with open DNA strands and DNA polymerase works to link the complementary nucleotide bases together growing strands through both condensation and hydroysis reactions. Through these mechanisms it is possible to target specific positions on the template DNA sequence that a scientist intends to amplify(PCR 1). When the PCR process is completed the targeted DNA sequence containing the single-nucleotide polymorphism (SNP) will have manufactured over a billion copies (amplicons). A SNP essentially is a type of gentic variation among organisms which represents a difference in a single nucleotide. For example, a SNP may replace a nucleotide cytosine (C) with a nucleotide thymine (T) in a certain part of an organisms DNA. These SNPs can be utilized as biological markers which in turn can help locate genes that have associative properties that contribute to the formation of harmful diseases. The targeted SNP for this research was rs17879961. This SNP is found in Humans (Homo sapiens) and represents a variation class SNV, which stands for single nucleotide variation. Furthermore, This SNP is a variant of the CHEK2 gene (Checkpoint kinase 2) which if present in a person's genome may increase their risk of developing breast cancer. This SNV signifies a single base change from a Thymine (T) to a Cytosine (C) located on chromosome 22 and its clinical significance is classified as a pathogenic allele. For example, this mutation would alter the normal alelle ATT and the middle position resulting the cancer associated allele ACT.
Primers for PCR Cancer allele forward primer: -> TTGAGAATG[TCA]CGTATGTAT Disease alleles will yield PCR products because the target amplicon is only associated with the cancer DNA sequences. Thus primer annealing will following base pairing rulese when it binds with the template strand. For example, triphosphate nucleotides align with open DNA strands and DNA polymerase works to link the complementary nucleotide bases together growing strands through both condensation and hydroysis reactions. The presence of a primer is required so that polymerase can proceed with directing the new nucleotides in place. Through these mechanisms it is possible to target specific positions on the template DNA sequence that a scientist intends to amplify. When the PCR process is completed the targeted DNA sequence containing the single-nucleotide polymorphism (SNP) will have manufactured over a billion copies (amplicons). Sources:
Our primers address the following design needs
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||