BME103 s2013:T900 Group2 L3: Difference between revisions
| Line 156: | Line 156: | ||
<!-- If your team decided to change the PCR and/or the Fluorimeter imaging protocols, summarize the new approaches/ features here and delete the '''We chose keep the protocols the same as the original system''' section. --> | <!-- If your team decided to change the PCR and/or the Fluorimeter imaging protocols, summarize the new approaches/ features here and delete the '''We chose keep the protocols the same as the original system''' section. --> | ||
'''We chose to include these new approaches/ features''' | '''We chose to include these new approaches/ features''' | ||
* | *We thought we would provide the PCR with an additional flourimeter apperatus (designed like a mini-box that a smart phone can be incorperated into) as well as the necessary chemicals. The user would have to provide some DNA sample (saliva or blood maybe) and we would provide an app for smart phone that the user buys. The app would in theory do all the necessary calculations in one easy step (pic->result), basically skipping the image j analysis. | ||
'''MATERIALS''' | '''MATERIALS''' | ||
'''Supplied In Kit Supplied by User''' | |||
<br> | |||
dNTPs Sample DNA | |||
<br> | |||
MgCl2 Forward and Reverse Primers | |||
<br> | |||
Reaction buffers Fluorescent, cancer-specific probe | |||
<br> | |||
Taq DNA Polymerase SYBR Green dye | |||
| Line 178: | Line 175: | ||
* '''PCR Protocol''' | * '''PCR Protocol''' | ||
<!-- Create a step-by-step procedure for setting up and running PCR reactions. Your instructions should include everything from adding reagents to the tubes, to programming the PCR machine and running the reaction.--> | <!-- Create a step-by-step procedure for setting up and running PCR reactions. Your instructions should include everything from adding reagents to the tubes, to programming the PCR machine and running the reaction.--> | ||
# | # The first step was to program the PCR machine and create the Thermal Cycler Program. The set-up used for the program is as follows | ||
# | '''Stage one:''' 1 cycle, 95 degrees Celsius for 3 minutes | ||
# | <br> | ||
'''Stage two:''' 35 cycles, 95 degrees Celsius for 30 seconds, 57 degrees Celsius for 30 seconds, 72 degrees Celsius for 30 seconds | |||
<br> | |||
'''Stage three:''' 72 degrees Celsius for 3 minutes | |||
<br> | |||
'''Final Hold:''' 4 degrees Celsius | |||
# The second step is to add reagents to the tubes. Start by gathering the necessary materials to set-up the DNA samples (pipette, PCR reaction mix, 8 transfer pipettes) | |||
# Set the pipette to 50 microliters | |||
# Place the transfer pipette onto the pipette to prevent cross contamination (never re-use). | |||
# | |||
# | |||
# | |||
# | |||
Revision as of 00:30, 16 April 2013
| Home People Lab Write-Up 1 Lab Write-Up 2 Lab Write-Up 3 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||
OUR TEAM
LAB 3 WRITE-UPOriginal System: PCR ResultsPCR Test Results
* Ave. INTDEN (Patient 91562)= 356035.3 *Ave. INTDEN (Patient 25235)= 452626.3
Bayes Theorem equation: P(A|B) = P(B|A) * P(A) / P(B)
Calculation 3: The probability that the patient will develop cancer, given a cancer DNA sequence.
New System: Design StrategyWe concluded that a good system Must Have:
We concluded that we would Want a good system to have:
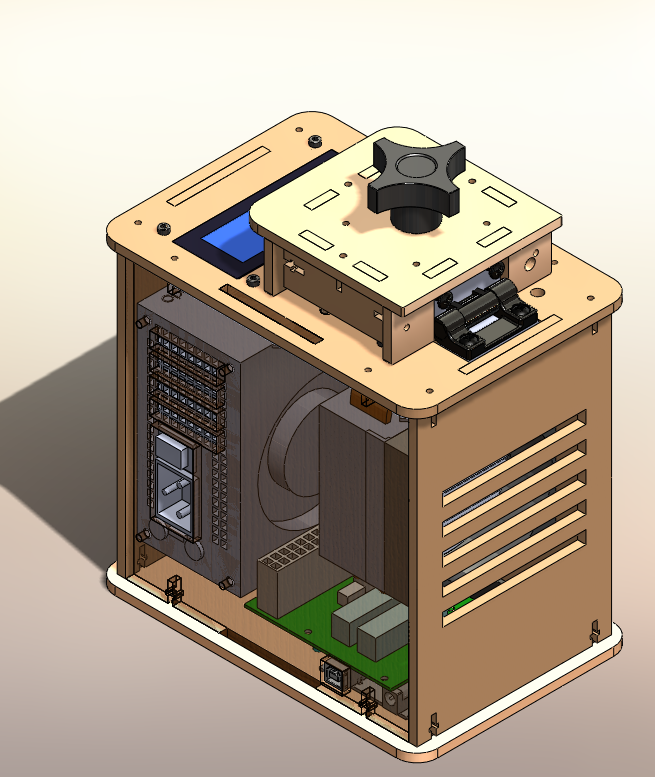
[[Image:
KEY FEATURES We chose to include these new features
[OR] We chose keep the devices the same as the original system
New System: ProtocolsDESIGN We chose to include these new approaches/ features
dNTPs Sample DNA
Stage one: 1 cycle, 95 degrees Celsius for 3 minutes
New System: Research and DevelopmentBACKGROUND Polymerase Chain Reaction (PCR) is a scientific method that utilizes DNA Polymerase to create a complimentary base strand from a template strand of DNA. Triphosphate nucleotides align with open DNA strands and DNA polymerase works to link the complementary nucleotide bases together growing strands through both condensation and hydroysis reactions. Through these mechanisms it is possible to target specific positions on the template DNA sequence that a scientist intends to amplify(PCR 1). When the PCR process is completed the targeted DNA sequence containing the single-nucleotide polymorphism (SNP) will have manufactured over a billion copies (amplicons). A SNP essentially is a type of gentic variation among organisms which represents a difference in a single nucleotide. For example, a SNP may replace a nucleotide cytosine (C) with a nucleotide thymine (T) in a certain part of an organisms DNA. These SNPs can be utilized as biological markers which in turn can help locate genes that have associative properties that contribute to the formation of harmful diseases. The targeted SNP for this research was rs17879961. This SNP is found in Humans (Homo sapiens) and represents a variation class SNV, which stands for single nucleotide variation. Furthermore, This SNP is a variant of the CHEK2 gene (Checkpoint kinase 2) which if present in a person's genome may increase their risk of developing breast cancer. This SNV signifies a single base change from a Thymine (T) to a Cytosine (C) located on chromosome 22 and its clinical significance is classified as a pathogenic allele. For example, this mutation would alter the normal alelle ATT and the middle position resulting the cancer associated allele ACT.
Primers for PCR Cancer allele forward primer: -> TTGAGAATG[TCA]CGTATGTAT Disease alleles will yield PCR products because the target amplicon is only associated with the cancer DNA sequences. Thus primer annealing will following base pairing rulese when it binds with the template strand. For example, triphosphate nucleotides align with open DNA strands and DNA polymerase works to link the complementary nucleotide bases together growing strands through both condensation and hydroysis reactions. The presence of a primer is required so that polymerase can proceed with directing the new nucleotides in place. Through these mechanisms it is possible to target specific positions on the template DNA sequence that a scientist intends to amplify. When the PCR process is completed the targeted DNA sequence containing the single-nucleotide polymorphism (SNP) will have manufactured over a billion copies (amplicons). Sources:
Our primers address the following design needs
New System: Software[THIS SECTION IS OPTIONAL. If your team has creative ideas for new software, and new software is a key component included in your new protocols, R&D, or machine design, you may describe it here. You will not receive bonus points, but a solid effort may raise your overall page layout points. If you decide not to propose new software, please delete this entire section, including the ==New System: Software== header.]
|
||||||||||||||||||||||||||||||||||||||||||