Banta:BetaRoll: Difference between revisions
Scott Banta (talk | contribs) No edit summary |
Kevin Dooley (talk | contribs) No edit summary |
||
| (10 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Banta_Lab}} | {{Template:Banta_Lab}} | ||
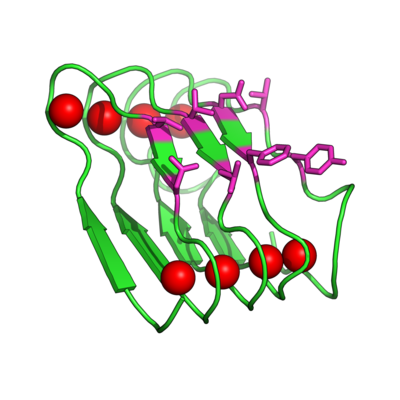
[[Image:BetaRoll.jpg|thumb|400px|right|Homology model of a folded Beta Roll domain where the red spheres indicate the bound Ca+2 ions and the purple resides are randomized for molecular recognition (Dooley, Kim, Lu, Tu, and Banta, 2012 Biomacromolecules).]] | |||
'''Evolving the Beta Roll Domain for Regulated Molecular Recognition''' | '''Evolving the Beta Roll Domain for Regulated Molecular Recognition''' | ||
Molecular recognition is ubiquitous in nature. Frequently antibodies are used in technology applications where biomolecular recognition is to be employed, but antibodies have several limitations in these applications, including difficulty in easily removing bound targets. This becomes especially important in the development of biosensors using | Molecular recognition is ubiquitous in nature. Frequently antibodies are used in technology applications where biomolecular recognition is to be employed, but antibodies have several limitations in these applications, including difficulty in easily removing bound targets. This becomes especially important in the development of biosensors using electrochemical-based signal transduction schemes. | ||
Instead of trying to engineer allosteric control into a molecular recognition molecule, we have started with intrinsically disordered scaffold, the beta roll domain, and we are working to evolve this allosterically regulated scaffold for biomolecular recognition. The naturally existing beta roll subdomain motif consists of tandem repeats of the sequence GGXGXDXUX, where U is an aliphatic amino acid and X is any amino acid. In the presence of calcium, the disordered peptide reversibly transitions to a beta roll spiral structure of two parallel beta sheet faces, where each beta strand has two solvent exposed variable residues | Instead of trying to engineer allosteric control into a molecular recognition molecule, we have started with intrinsically disordered scaffold, the beta roll domain, and we are working to evolve this allosterically regulated scaffold for biomolecular recognition. The naturally existing beta roll subdomain motif consists of tandem repeats of the sequence GGXGXDXUX, where U is an aliphatic amino acid and X is any amino acid. In the presence of calcium, the disordered peptide reversibly transitions to a beta roll spiral structure of two parallel beta sheet faces, where each beta strand has two solvent exposed variable residues. | ||
We have characterized a native beta roll subdomain with various end-capping groups in order to identify a minimal calcium-responsive beta roll unit. We believe that the beta roll faces are suitable binding surfaces and that calcium-induced structure formation can be used as a mechanism to control the formation of the engineered biomolecular recognition interface. | We have characterized a native beta roll subdomain with various end-capping groups in order to identify a minimal calcium-responsive beta roll unit. We have immobilized the beta roll on surfaces, and we have developed a FRET-based system to monitor structural perturbations. We have truncated and concatenated the beta roll and we have discovered beta roll sequences that can reversibly precipitate which is useful for protein purification. We believe that the beta roll faces are suitable binding surfaces and that calcium-induced structure formation can be used as a mechanism to control the formation of the engineered biomolecular recognition interface. To test this, we have engineered beta rolls to dimerize in a calcium-dependent manner and used these to create cross-links for protein hydrogels. We have randomized one face of the beta roll unit and we are using directed evolution to identify beta roll peptides with biomolecular recognition capabilities. | ||
'''Related Publications''' | '''Related Publications''' | ||
<biblio> | <biblio> | ||
#Paper9 pmid=23642248 | |||
#Paper8 pmid=23581466 | |||
#Paper7 pmid=23173179 | |||
#Paper6 pmid=22545587 | |||
#Paper5 pmid=21416544 | |||
#Paper4 pmid=20438736 | |||
#Paper3 pmid=19860484 | #Paper3 pmid=19860484 | ||
#Paper2 pmid=17376876 | #Paper2 pmid=17376876 | ||
#Paper1 pmid=17450770 | #Paper1 pmid=17450770 | ||
</biblio> | </biblio> | ||
Revision as of 12:39, 14 August 2014

Banta Lab
Protein and Metabolic Engineering
| Home | Lab Members | Publications | Research Interests | Courses | Pictures | Positions Available |
Evolving the Beta Roll Domain for Regulated Molecular Recognition
Molecular recognition is ubiquitous in nature. Frequently antibodies are used in technology applications where biomolecular recognition is to be employed, but antibodies have several limitations in these applications, including difficulty in easily removing bound targets. This becomes especially important in the development of biosensors using electrochemical-based signal transduction schemes.
Instead of trying to engineer allosteric control into a molecular recognition molecule, we have started with intrinsically disordered scaffold, the beta roll domain, and we are working to evolve this allosterically regulated scaffold for biomolecular recognition. The naturally existing beta roll subdomain motif consists of tandem repeats of the sequence GGXGXDXUX, where U is an aliphatic amino acid and X is any amino acid. In the presence of calcium, the disordered peptide reversibly transitions to a beta roll spiral structure of two parallel beta sheet faces, where each beta strand has two solvent exposed variable residues.
We have characterized a native beta roll subdomain with various end-capping groups in order to identify a minimal calcium-responsive beta roll unit. We have immobilized the beta roll on surfaces, and we have developed a FRET-based system to monitor structural perturbations. We have truncated and concatenated the beta roll and we have discovered beta roll sequences that can reversibly precipitate which is useful for protein purification. We believe that the beta roll faces are suitable binding surfaces and that calcium-induced structure formation can be used as a mechanism to control the formation of the engineered biomolecular recognition interface. To test this, we have engineered beta rolls to dimerize in a calcium-dependent manner and used these to create cross-links for protein hydrogels. We have randomized one face of the beta roll unit and we are using directed evolution to identify beta roll peptides with biomolecular recognition capabilities.
Related Publications
- Banta S, Dooley K, and Shur O. Replacing antibodies: engineering new binding proteins. Annu Rev Biomed Eng. 2013;15:93-113. DOI:10.1146/annurev-bioeng-071812-152412 |
- Shur O, Dooley K, Blenner M, Baltimore M, and Banta S. A designed, phase changing RTX-based peptide for efficient bioseparations. Biotechniques. 2013 Apr;54(4):197-8, 200, 202, 204, 206. DOI:10.2144/000114010 |
- Shur O and Banta S. Rearranging and concatenating a native RTX domain to understand sequence modularity. Protein Eng Des Sel. 2013 Mar;26(3):171-80. DOI:10.1093/protein/gzs092 |
- Dooley K, Kim YH, Lu HD, Tu R, and Banta S. Engineering of an environmentally responsive beta roll peptide for use as a calcium-dependent cross-linking domain for peptide hydrogel formation. Biomacromolecules. 2012 Jun 11;13(6):1758-64. DOI:10.1021/bm3002446 |
- Shur O, Wu J, Cropek DM, and Banta S. Monitoring the conformational changes of an intrinsically disordered peptide using a quartz crystal microbalance. Protein Sci. 2011 May;20(5):925-30. DOI:10.1002/pro.625 |
- Blenner MA, Shur O, Szilvay GR, Cropek DM, and Banta S. Calcium-induced folding of a beta roll motif requires C-terminal entropic stabilization. J Mol Biol. 2010 Jul 9;400(2):244-56. DOI:10.1016/j.jmb.2010.04.056 |
- Szilvay GR, Blenner MA, Shur O, Cropek DM, and Banta S. A FRET-based method for probing the conformational behavior of an intrinsically disordered repeat domain from Bordetella pertussis adenylate cyclase. Biochemistry. 2009 Dec 1;48(47):11273-82. DOI:10.1021/bi901447j |
- Chockalingam K, Blenner M, and Banta S. Design and application of stimulus-responsive peptide systems. Protein Eng Des Sel. 2007 Apr;20(4):155-61. DOI:10.1093/protein/gzm008 |
- Banta S, Megeed Z, Casali M, Rege K, and Yarmush ML. Engineering protein and peptide building blocks for nanotechnology. J Nanosci Nanotechnol. 2007 Feb;7(2):387-401. DOI:10.1166/jnn.2007.153 |