Berk2006-ConjugationTeam2: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
------------- | ------------- | ||
== SIL_07/31/06 == | |||
Today, Matt miniprepped 25 promoters from the library and also made -80 stocks of them. <br>. | |||
I miniprepped the TraG and TraM complements with double terminators pSB1AKG0-J23026 and pSB1AKG0-J23027. Then I digested them in EcoR/Pst and mapped them to check if they are the product desired (wouldhave 2 bands) or if they are the parent vector (would have 3 bands). <br> | |||
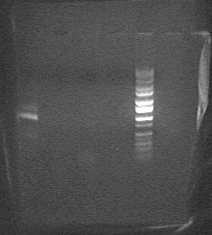
'''Picture of gel from EcoR1/Pst1 digest (lane 1 = pSB1AKG0-J23026 (band1) clone 1, lane 2= pSB1AKG0-J23026 (band1) clone2, lane 3 = pSB1AKG0-J23026 (band2) clone 1, lane 4 = pSB1AKG0-J23026 (band2) clone2, lane 5 = pSB1AKG0-J23026 clone 1, lane 6 = pSB1AKG0-J23026 clone2, lane 7 = ladder) '''<br> | |||
[[Image:SIL073106.jpg]]<br> | |||
I also added the double terminator to the trbC complement by ligation and transformation. <br> | |||
Also, Jen PCRed the oligos of the TraG KO 20mers and is growing up saturated cultures of the pOX38 knockouts of trbCf and OriTf. <br> | |||
== SIL_07/27/06 == | == SIL_07/27/06 == | ||
Today, Matt did the knockouts of OriT and trbCf on the F plasmid (both poX38xpKD46 variants GeneHog and MC1061). He also did some library stuff.<br> | Today, Matt did the knockouts of OriT and trbCf on the F plasmid (both poX38xpKD46 variants GeneHog and MC1061). He also did some library stuff.<br> | ||
Revision as of 12:36, 31 July 2006
Return to main page
SIL_07/31/06
Today, Matt miniprepped 25 promoters from the library and also made -80 stocks of them.
.
I miniprepped the TraG and TraM complements with double terminators pSB1AKG0-J23026 and pSB1AKG0-J23027. Then I digested them in EcoR/Pst and mapped them to check if they are the product desired (wouldhave 2 bands) or if they are the parent vector (would have 3 bands).
Picture of gel from EcoR1/Pst1 digest (lane 1 = pSB1AKG0-J23026 (band1) clone 1, lane 2= pSB1AKG0-J23026 (band1) clone2, lane 3 = pSB1AKG0-J23026 (band2) clone 1, lane 4 = pSB1AKG0-J23026 (band2) clone2, lane 5 = pSB1AKG0-J23026 clone 1, lane 6 = pSB1AKG0-J23026 clone2, lane 7 = ladder)

I also added the double terminator to the trbC complement by ligation and transformation.
Also, Jen PCRed the oligos of the TraG KO 20mers and is growing up saturated cultures of the pOX38 knockouts of trbCf and OriTf.
SIL_07/27/06
Today, Matt did the knockouts of OriT and trbCf on the F plasmid (both poX38xpKD46 variants GeneHog and MC1061). He also did some library stuff.
Jenn minipreped, mapped, and sequenced the trbCf complement pSB1A2-J23014.
Here is the gel from the trbCf complement analytic digest (lane 1 = ladder, lane 2 = clone 1, lane 3=clone2)

I am growing up overnights of pSB1AKG0-J23026 and pSB1AKG0-J23027 the TraG and TraM complements with double terminators so that they can be miniprepped and sequenced. Unfortunately, when I was making pSB1AKG0-J23026 the digest resulted in more bands that expected, but I cut out ones that seemed to be at appropriate lenghts and thus we have 2 variants of the plasmid which need to be sequenced in order to determine which one is the correct one.
Also finally, I transformed the helper plasmid pCP20 into J23017 (Rlambda TraG KO) and plated it on Amp Cam at 30 degrees. I had to do a small scale electroporation prep to make the competent cells in order to do this.
MF&JL_07/26/06
We picked colonies, one from each of the plates, of the four plates that we plated yesterday from the conjugations( pOX38 w/ pKD46 in Gene Hogs conjugated at 30 degrees, pOX38 w/ pKD46 in MC1061 conjugated at 30 degrees, pOX38 w/ pKD46 in Gene Hogs conjugated at 37 degrees and pOX38 w/ pKD46 in MC1061 conjugated at 37 degrees).
Sam grew up colonies of trbC compliments so she can mini prep them on Thursday. She also grew up the Rlambda w/ [TraG] KO (JO23017) from the plate that we streaked yesterday.
Picture of the map of clones 7A, 7B, 8A, and 8B. (in that order)
I did the PCR and maping of clones 7A, 7B, 8A, and 8B again. The second time, no bands were seen.
MF&SIL&JL_07/25/06
We have conjugated the pOX38 with some of Chris's stocks of pKD46 (in GeneHogs and MC1061) . We will have to see tomorrow if his stocks were still healthy.
Also, the trbCf complement PCR worked today (affirmed by a gel) and we have cleaned up the PCR, digested it in dPN1, EcoRI, and SpeI, then cleaned up the digestion, ligated with pSB1A2-I13511 digest in ecoRI and SpeI, and then transformed it into TG1 cells.
The sequencing data came back for the TraM and TraG complements and they are positive for both, so they are tough tagged and put in the freezer.
Finally, we are doing genomic minipreps of the RlambdaTraGknockout clones, from here on known as J23017. We then PCRed them with JL10 and JL11 oligos and hopefully when we map them, they'll show a band around 1200, instead of 2000 like last time, which indicated wild type Rlambda.
Here is the Rlambda traG KO PCR's Clones 6,7,8:

Clones 7 and 8 seem to have the correct band of the TraGr knockout, but they also seem to have the wt TraGr. Therefore, we restreaked clone 8 onto a Cm plate to try to seperate the wildtype from the knockout. Also, we did four more PCR preps (fusion) for clones 7 and 8. This time we used oligos JL10 and 1016R (ie. 7A, 8A), and we used oligos 824 and JL11 (ie. 7B, 8B). We did this in order to make sure we have the TraGr knockout.
We are also adding double terminators from pSB1AKG0-B0015 digested in Eco/Xba to the TraM and TraG biobricks by digesting them in Eco and SpeI.
MF_07/24/06
Today we started cultures of pOX38 and pKD46 (in GeneHogs and MC1061). The pKD46 was from Chris's -80 stock. We are redoing the mating and the knockout because pOX38 mated with pKD46 aquired Cloramphenicol sensitivity somewhere in our procedure. Also, our four plates that we plated on Friday gave us unexpected results. All four plates came out with yellow colonies and smell like their is some kind of bacteria. We believe that DH10B is the culprit in this instance. We think that DH10B might have been contaminated before we began our experiment.
However, we are proceeding with the Rlambda TraG knockout by doing a genomic miniprep of the 4 cultures we grew up Friday, then PCRing with the TraG complement oligos JL10 and JL11, and finally mapping them to see if the size of the PCR fragment can give us a conclusion on whether TraG has been knocked out.
Also, the parts registry has been updated with all the complements and knockouts.
We are still waiting for out the sequencing data for the TraM and TraG complements.
We got our trbCf 20mers! So we are PCRing them with Olambda as the DNA template.
Mapping of cultures that we started on Friday was not successful. Gel electrophoresis showed three failures and one WT traGr. So, we are growing eight more over night cultures of J23017 [traGr] from Rlambda.