Berk2010-Daniela: Difference between revisions
No edit summary |
No edit summary |
||
| (81 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==[[User:Daniela Mehech|Daniela Mehech]] 18:29, 13 August 2010 (EDT)== | |||
Goal Parts RE map<br> | |||
[[Image:GoalPartsA.jpg|300px]]<br> | |||
[[Image:GoalPartsB.jpg|300px]]<br> | |||
<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 21:17, 12 August 2010 (EDT)== | |||
Tuesday (8/10): <br> | |||
Transformed all goal parts.<br> | |||
Paused assembly on 4 remaining parts<br> | |||
<br> | |||
Wednesday (8/11):<br> | |||
Picked 2 colonies for all goal parts<br> | |||
did not pick colonies for Igem 107 in CA<br> | |||
<br> | |||
Thursday (8/12):<br> | |||
Mini-prepped all goal parts except 4<br> | |||
Picked colonies for B3 in plates A and B, and C3 and D3 in plate A<br> | |||
<br> | |||
To Do Friday:<br> | |||
Mini-prep those 4 parts<br> | |||
RE map everything<br> | |||
<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:34, 9 August 2010 (EDT)== | |||
Friday (8/6):<br> | |||
Threw out 107, 109, 115, 116 because we plated them on the wrong antibiotics. Need to redo their assembly | |||
mini-prep, RE, sequence 130, 135<br> | |||
Add terminator to 112, 113<br> | |||
<br> | |||
Saturday (8/7):<br> | |||
pick colonies for 107, 109, 112, 113, 115, 116, 126 <br> | |||
<br> | |||
Sunday (8/8):<br> | |||
mini-prep 107, 109, 112, 113, 115, 116, 126<br> | |||
<br> | |||
Monday (8/9):<br> | |||
Assemble 128, 129 <br> | |||
Transfer 107 to CA (in order to assemble 127)<br> | |||
Plate 140 on arabinose<br> | |||
Transform 112, 113, 115, 116 into lefties (we were using righty methylated minipreps when trying to add the terminator)<br> | |||
<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 19:25, 5 August 2010 (EDT)== | |||
To Do Thursday:<br> | |||
Analyze sequencing data<br> | |||
Mini-prep, RE, sequence 124, 125, 134, E/B transfer<br> | |||
Transform and plate 125, 126<br> | |||
Assemble and transform GFP with ig114 <br> | |||
Redo 107, 109, 112, 113, 115, 116, 130, 135<br> | |||
<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 19:33, 4 August 2010 (EDT)== | |||
Mini-prep and Map Catch ups | |||
send in sequencing 133, 136, 138 <br> | |||
pick colonies 124, 134, E/B transfer for ig114 <br> | |||
Manual Assembly 125, 126 <br> | |||
Add terminator 107, 109, 115, 116 <br> | |||
regular assembly 130, 135, 112, 113 <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:15, 3 August 2010 (EDT)== | |||
August 2nd: <br> | |||
Most of Stage 4 parts come out with good sequencing.<br> | |||
Problems:<br> | |||
ig114 has two bp mutations that got passed on to just about every single part. The mutations shouldn't affect the function but we're going to test that to make sure<br> | |||
b1006! is missing from various parts because of iGEM 66 failure. We're going to add those to the ends of the parts<br> | |||
A couple of parts did not work and we can't figure out why. We'll remake their parent parts and try again<br> | |||
igem111 that was in Laeretes was wrong. We put in a different copy from Gandalf in Laeretes that sequenced correctly. We're re doing those parts<br> | |||
igem 92 was wrong. We're redoing it and those parts<br> | |||
<br> | |||
Transformed the parts we need to remake<br> | |||
<br> | |||
August 3: | |||
Picked Colonies for parts that transformed<br> | |||
Three parts didn't grow so we made them by hand. Not sure if the bands in the gel came out the right size<br> | |||
<br> | |||
We have 11 sequence confirmed goal parts! | |||
<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 19:53, 30 July 2010 (EDT)== | |||
July 28: <br> | |||
Mini-prepped and did RE map<br> | |||
RE map came out bad for almost all of Stage 4 parts. For some reason a lot of parts were not being added.<br> | |||
Sent in Stage 2 and Stage 3 parts that had small parent parts (because RE mapping isn't sensitive enough to show us if small parts were assembled correctly)<br> | |||
Picked more colonies from Stage 4<br> | |||
Re-did stage 4 and included some Stage 4 Catchups<br> | |||
<br> | |||
July 29: <br> | |||
Mini-prepped Stage 4 Round 1 re-picks<br> | |||
Picked colonies for Stage 4 Round 2<br> | |||
Analyzed sequencing results - apparently iGEM 66 never took on it's righty parent part and everything that came from igem 66 is missing the terminator<br> | |||
<br> | |||
July 30:<br> | |||
Mini-prepped Stage 4 round 2<br> | |||
Did RE map of Stage 4 round 1 re-picks and Stage 4 round 2<br> | |||
RE map is still questionable so sent in all of Stage 4 that we had for sequencing<br> | |||
<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:55, 27 July 2010 (EDT)== | |||
July 26: Did stage 4 assembly and Stage 3 Catchups<br> | |||
July 27: Most things grew except for igem119 and igem104. igem113 sequenced wrong so needs to be redone<br> | |||
Picked colonies for things that grew, but did not start catchups just yet<br> | |||
<br> | |||
===To Do=== | |||
Mini-prep everything<br> | |||
Do Stage 3 cathcups (106 and 104) and Stage 4 catch ups that we can do without 106 and 104 (about 6 out of 10)<br> | |||
sequence some goal parts<br> | |||
Do last of stage 4 catchups<br> | |||
Assay!<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 14:46, 23 July 2010 (EDT)== | |||
July 22: Mini-prepped re-do's of stage 1 and what we had for stage 2. RE map came out good (see Christoph's notebook). We made a plate of all good Stage 1 and Stage 2 parts. <br> | |||
<br> | |||
Picked colonies for sbb18, igem64 and igem65. These still need to be added to our final plate<br> | |||
<br> | |||
Today: Mini-prep and RE map sbb18, igem64 and igem65, and added them to final plate. See RE map below<br> | |||
[[Image:Stage0and1Catchups.jpg|300px]]<br> | |||
<br> | |||
Did Stage 3 and stage 2 assembly catchups | |||
==[[User:Daniela Mehech|Daniela Mehech]] 19:12, 21 July 2010 (EDT)== | |||
Most of Stage 2 parts grew although very sparsely. Picked colonies for them, will mini-prep tomorrow morning. We will re-do the 4 missing parts when we get RE map and co-transformation plate results, that way we only have one catch-up group <br> | |||
<br> | |||
Picked colonies for stage 1 re-transformations. They all grew except for iGEM65 because we used DNA from the wrong well. Transformed iGEM65 again using DNA from Rosencratz<br> | |||
<br> | |||
iGEM64 did not grow with stage 2. Doing assembly by hand (separate digests, gel purified, zymo column). We're re-transforming sbb18 in AK from Mad Hatter to try to get a better mini-prep. If manual assembly still doesn't work we'll have to try again with new sbb18 mini-preps | |||
===To Do=== | |||
Mini-prep stage 2 and stage 1 parts. RE map.<br> | |||
Make new plates for final stage 1 and 2 parts.<br> | |||
Do Stage 2 catch-ups<br> | |||
Pick colonies for 65,64 and sbb18<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 14:48, 20 July 2010 (EDT)== | |||
Christoph picked colonies, mini-prepped, ran RE on Stage 1 parts and did the catchups. Check his page for gel pictures. We have all the stage 1 parts except for one where we used the wrong parent part twice. We did Stage 2 on yesterday. Today none of the rigthies grew and 5 of the 6 Lefties grew.<br> | |||
<br> | |||
possible sources of error:<br> | |||
-put antibiotics in wrong location on robot<br> | |||
-Ophelia layout is wrong<br> | |||
-DNA was too dilute<br> | |||
-Cells were too dilute<br> | |||
<br> | |||
to do: <br> | |||
-check Ophelia's layout<br> | |||
-col PCR on things that did grow<br> | |||
-Re do stage 2 with higher DNA and Cell concentration<br> | |||
Colony PCR of what did grow from Stage 2 Round 1:<br> | |||
[[Image:Stage2Round1ColPCR.jpg|300px]]<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 23:27, 12 July 2010 (EDT)== | |||
July 10: Broke my back; will return to lab later this week. Christoph doing project solo for now <br> | |||
July 9: Transformed cells and plated them <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 16:46, 8 July 2010 (EDT)== | |||
July 7: Picked 4 colonies for each part. Re-made 7 parts that did not grow well/at all <br> | |||
Sent 7 with 10 to sequencing<br> | |||
<br> | |||
Today:<br> | |||
66% of parts had all 4 colonies co-transformed. We've gone back to troubleshooting<br> | |||
7 with 10 sequenced perfectly, but we've left them in the tubes instead of moving them to the plate<br> | |||
<br> | |||
Troubleshooting:<br> | |||
Doing a RE digest to see if parts are methylated correctly (these are all lefties):<br> | |||
[[Image:Stage1TestingMethCorrectDigest.jpg|300px]]<br> | |||
Another RE digest:<br> | |||
[[Image:BamBglDigestOnRightyAndLefty.jpg|300px]]<br> | |||
<br> | |||
Triple Digest<br> | |||
[[Image:TripleDigest.jpg|300px]]<br> | |||
<br> | |||
We began assembly again, this time with more dilute DNA to see if this helps reduce co-transformation. We did digestion and left the ligation over night<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 21:29, 6 July 2010 (EDT)== | |||
RE mapping of 3 parts that we repicked (6 with 24 in AK, KA and 7 with 24). They all came out faint, but most look good)<br> | |||
[[Image:REofRepickedParts.jpg|300px]] | |||
Did stage 1 assembly without a problem. | |||
Picked Colonies for 7 with 10. Colony PCR results shown below; they all look good <br> | |||
[[Image:7_with_10_redo_col_PCR.jpg|300px]] | |||
===To Do=== | |||
Check colony PCR result for 7 with 10, mini-prep | |||
pick colonies, colony PCR, streak and inoculate for stage 1 assembly | |||
==[[User:Daniela Mehech|Daniela Mehech]] 16:42, 5 July 2010 (EDT)== | |||
Re-digested parts that showed up faint or not digested:<br> | |||
[[Image:Stage 0 mehtylated RE map reruns.jpg|300px]]<br> | |||
Everything came out good the second time with the exception of 7 with 10. We noticed that we've been using a mini-prep of 7 with 10 that had a bad sequencing result. We did a lefty transformation of the 7 with 10 that mini-prepped correctly <br> | |||
<br> | |||
We chose to repick colonies from 4 parts that did not show up correctly on the RE map at all. | |||
===To Do=== | |||
Pick colonies from 7 with 10 | |||
Mini-prep new colonies | |||
==[[User:Daniela Mehech|Daniela Mehech]] 15:32, 2 July 2010 (EDT)== | |||
Only one colony from the manual assembly (out of 32) was not co-transformed (the same one that had the correct band in the colony PCR gel)<br> | |||
RE map of Methylated Parts for Stage 0:<br> | |||
*Lefty methylations:<br> | |||
**[[Image:Stage_0_mehtylated_RE_map_part_1A.jpg|500px]] | |||
**[[Image:Stage_0_mehtylated_RE_map_part_1B.jpg|500px]] | |||
*Righty Methylations: <br> | |||
**[[Image:Stage_0_mehtylated_RE_map_part_2A.jpg|500px]] | |||
**[[Image:Stage_0_mehtylated_RE_map_part_2B.jpg|500px]] | |||
*Extra Righties: <br> | |||
**[[Image:REmapColum7.JPG|300px]]<br> | |||
===To Do=== | |||
Decide from RE map gel which parts need to be re-digested, re-picked or re-transformed | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:27, 1 July 2010 (EDT)== | |||
[[Image:ColPCRiGEM64manualAssembly.JPG|300px]]<br> | |||
Colony PCR of igem10_064 when Christoph and I made it by hand. Only one lane has a band at 1200. We mini-prepped all of them using the 8-strip method.<br> | |||
<br> | |||
We picked colonies for all of our new methylated strains. Except for B4, nothing grew on that so we transformed | |||
===To Do=== | |||
Mini-prep methylated parts | |||
RE map and analyze gel. Make plate with only correct parts that have been confirmed by RE mapping | |||
Pick colonies for B4, mini-prep at end of the day | |||
Check for co-transformation | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:48, 30 June 2010 (EDT)== | |||
We used the wrong plate layout when we were remaking our parts - none of them work and we had to redo them. <br> | |||
Our co-transformation experiment didn't work (next to nothing grew), so we're changing it and doing it again. <br> | |||
We decided to start over from our unmethylated plate - we transformed every part into Righty/Lefty strains <br> | |||
===To Do=== | |||
pick colonies from re-made parts. Mini-prep at end of the day<br> | |||
pick colonies from co-transformation experiment and streak for triple resistance. Mini-prep at end of the day<br> | |||
Mini-prep the one part that was correct (6 with 23) and RE map it <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:42, 29 June 2010 (EDT)== | |||
All 16 colonies of all 3 parts that we picked were co-transformed. No matter how many times we do it, either manually or with the robot we keep getting a high rate of co-transformation. We're going to test transforming with different concentrations of DNA, different amounts of cells, and a higher concentration of enzyme. <br> | |||
<br> | |||
We transformed more parts (those that we are still missing and that did not have enough volume in plate B). They should be done by Wednesday, then we can do robot assembly on Thursday. By Thursday then maybe our experiment will we show us what concentration of DNA/cell/enzymes work best to minimize co-transformations <br> | |||
<br> | |||
===To Do=== | |||
Check all of experimental parts (igem10_072) for co-transformation (pick colonies and streak) <br> | |||
Pick colonies, mini-prep, RE map and add newly made parts to dilution plate <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 19:13, 28 June 2010 (EDT)== | |||
Began making dilution plate - transferred 20 ul of parts from either plate B or plate A (whichever had better RE map). Some parts though did not have enough volume in plate B and hadn't been RE mapped in plate A. We're just going to re-transform a big patch of parts so we know for sure that they're correct and that we have enough volume. <br> | |||
<br> | |||
Our sequences for vectors pmLL8 and pmLL9 were switched, which explains why we thought we had so many parts wrong. Almost all of the parts in plate B are correct but we can't use half of them because we don't have enough volume. We're not using parts from plate A that were not RE mapped either. We're re-transforming parts and re making them. Now that we've determined that for the most part we were using the correct parts we need to keep looking for an explanation of why our stage 1 parts did not come out right.<br> | |||
<br> | |||
Tim picked colonies for us from the parts that re-transformed on Friday. We mini-prepped them and RE mapped. Now that we corrected our sequences all the mini-preps came out correct and we added the newly-made parts to our dilution plate. | |||
<br> <br> | |||
We picked colonies from the composite parts we're making by hand. At the end of the day we could tell everything was co-transformed except for igem10_076. It only had 5 colonies grow. We mini-prepped all 5 colonies and picked 16 more colonies for the three other parts. <br> | |||
===To Do=== | |||
re-make parts that we did not have enough volume of in plate A and that we just don't have at all (7 with 10)<br> | |||
Check for co-transformation in manual parts. RE map igem10_076 to determine if it is correct <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 17:54, 25 June 2010 (EDT)== | |||
Transformed 7 unmethylated parts into Righty/Lefty strain. Left them in incubator for someone to remove them tomorrow<br> | |||
Did manual assembly of 4 parts (the 4 goal parts in stage 1, but we transformed them into Righty)<br> | |||
Made plate layouts and wrote CSV files in preparation for robotic assembly. We are not going to having any mislabeled wells this time<br> | |||
===To Do=== | |||
Pick colonies and mini-prep (Monday)<br> | |||
Transfer DNA to new stock plate (Monday)<br> | |||
RE Map all of new plate and sequence some parts to ensure that everything is where it should be (Monday/Tuesday)<br> | |||
Repeat Stage 1 Robotic 2A assembly (Tuesday/Wednesday)<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 18:42, 24 June 2010 (EDT)== | |||
Most of plate B mapped correctly. We mapped the parts from plate A that mapped incorrectly on plate B: <br> | |||
[[Image:REmappingPlateAPartial.JPG|300px]]<br> | |||
===To Do=== | |||
Re-make those parts that mapped incorrectly in both plate A and B (just transform unmethylated parts into Righty or Lefty strain) | |||
Do manual 2AB assembly of four parts to determine if the procedure works, at least by hand | |||
Make new methylated stock plate (take best parts from plate A, B and the new ones that we make) | |||
==[[User:Daniela Mehech|Daniela Mehech]] 16:23, 23 June 2010 (EDT)== | |||
We are going to start over with our mehtylated parts so we ran a RE-mapping of our original methylated parts plate to determine if that plate is correct.<br> | |||
Restriction map of methylated parts Plate B: <br> | |||
Part 1: <br> | |||
[[Image:Re_map_methylated_plate_A_part_1.jpg|500px]]<br> | |||
Part 2:<br> | |||
[[Image:Re map methylated plate A part 2.jpg|500px]]<br> | |||
<br> | |||
All the colonies I repicked were co-transformed, so I didn't run the ColPCR gel<br> | |||
<br> | |||
RE mapping of random sampling of methylated and unmethylated stocks. Most are right, but a few came out wrong | |||
[[Image:REmappingUnmethANDMethStocks.JPG|300px]]<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 20:34, 22 June 2010 (EDT)== | |||
Mini-prepped selected parts from groups 3 and 4. Some parts were co-transformed/colony PCR showed no good clones. I repicked more colonies for those parts. Did a restri | |||
ction map of the mini-prepped parts. Everything came out with 3 bands. Maybe the digestion did not go to completion.<br> | |||
<br> | |||
Because we are getting such a high failure rate Christoph and I started looking over our entire procedure to see if something wrong happened at the beginning. A RE-mapping of our methylated parts plate did not perfectly match our plate layout = somewhere something went wrong and we need to start over <br> | |||
<br> | |||
[[Image:Restriction_map_of_methylated_parts_rows_A_and_E.jpg|500px]]<br> | |||
The top gel is RE-mapping of randomly selected methylated parts. The bottom gel is RE-mapping of mini-prepped composite parts | |||
===To Do=== | |||
Look at Restriction Mapping results - decide what to re-transform and what to send for sequences and what to deem finished | |||
Look over repicked results (run colony PCR and check co-transformation plate) - decide what to mini-prep and what to retransform | |||
RE-map unmethylated parts to see if at least that is correct | |||
==[[User:Daniela Mehech|Daniela Mehech]] 14:19, 21 June 2010 (EDT)== | |||
Colony PCR results from Group 2 <br> | |||
[[Image:colonyPCRredoneparts.JPG|300px]]<br> | |||
lane 1,3,5,7 = 5 w/ 35 L <br> | |||
lane 2,4,6,8 = 5 w/ 38 <br> | |||
lane 9,11,13,15 = 5 w/ 35 R <br> | |||
lane 10,12,14,16 = 6 w/33 L<br> | |||
lane 17,19,21,23 = 6 w/33 R<br> | |||
lane 18,20,22,24 = 9 w/ 8 L<br> | |||
lane 25, 27,29,31 = 7 w/40<br> | |||
lane 26,28,30,32 = 9 w/ 34 L<br> | |||
lane 33,34,35,36 = 9 w/ 8 R<br> | |||
lane 37,38,39,40 = 9 w/ 34 R<br> | |||
Except for first 8 lanes, everything looked good and we mini-prepped two of each. Mini-prepped lanes 1-8 too and will send them for sequencing<br> | |||
Colony PCR results for group 3:<br> | |||
[[Image:colonyPCRforgottenparts.JPG|300px]]<br> | |||
Loaded as: 1,9,2,10,3,11,4,12,5,13,6,14,7,15,8,16,17,25,18,26,19,27,20,28,21,29,22,30,23,31,24,32 | |||
Colony PCR results for group 4:<br> | |||
[[Image:colonyPCRrepickedcolonies.JPG|300px]]<br> | |||
Restriction Mapping of everything we've mini-prepped (groups 1 and 2)<br> | |||
[[Image:REmappingStage1Round1RightHalf.JPG|300px]]<br> | |||
Right Half<br> | |||
[[Image:REmappingStage1Round1LeftHalf.JPG|300px]]<br> | |||
Left Half<br> | |||
===To Do=== | |||
Check for co-transformation for Groups 3 and 4 <br> | |||
Mini-Prep good colonies of group 3 and 4 <br> | |||
Send to sequencing some parts? <br> | |||
Begin Stage 2 (hopefully all our stage 1 parts are right) <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 21:28, 18 June 2010 (EDT)== | |||
We labeled our transformation plate wrong so when we went to plate the cells many of them were plated on the wrong antibiotics. We still had colonies grow because they were co-transformed and had resistance to all antibiotics. This also explains why so many colonies grew when we tested for co-transformation. We have split our stage 1 parts into 4 groups: <br> | |||
1. Colonies grew on proper antibiotics and were not co-transformed. There are 12 of these. We mini-prepped these today and will do restriction mapping properly on Monday<br> | |||
2. Colonies that grew on wrong antibiotics need to be redone. There are 10 of these.We digested, ligated and transformed them today. <br> | |||
3. Parts that we thought we hadn't done (see yesterday's notes), but it turns out we did do them without realizing it since our plates were labeled wrong. Since we did them twice and they were all plated correctly the second time we will pick colonies, do Colony PCR, check for co-transformation and mini-prep these 9 on Monday.<br> | |||
4. Colonies that grew on the right antibiotics but were co-transformed. We will pick more colonies from the original plate to do ColPCR<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 18:37, 17 June 2010 (EDT)== | |||
We got cell growth for every part we plated (although in some parts there were very few colonies, about 3 or 4).<br> | |||
We made a Colony PCR Mastermix and tried to distribute it to two PCR plates with the robot but the robot kept giving us an error message (It said it couldn't pipette 19uL if there was 17uL in the tube but there was 2 ml in the tube and we wanted it to pipette 17uL). We gave up and I pipetted all 136 wells by hand. <br> | |||
Christoph and I picked colonies, added them to the PCR plate, innoculated them in a 96-well plate and left them in the incubator. We ran a ColPCR3K program on the both PCR plates. <br> | |||
Christoph noticed that we had based all of our csv robot files from the wrong list. We made all of the parts we needed but some of the parts had to be inserted into multiple vector backbones. We began making the 6 forgotten parts by hand. 3 of these parts need to be transformed into both Righties and Lefties so we're making 9 parts by hand.<br> | |||
We dabbed broth from our 96-well inoculated plate on both AKC plates and LB-only plates as negative and positive controls respectively for whether we picked any co-transformed colonies.<br> | |||
We need to run a huge gel when the PCR finishes at 4:40pm. Then we need to analyze the results. Christoph already named all the new composite parts and calculated their sizes.<br> | |||
===To Do=== | |||
We will mini-prep our good colonies tomorrow. Hopefully we'll have at least two good copies for each part so that we can continue with Plates A and B for stage 2. <br> | |||
We can start planning for Stage 2 assembly when we have free time. If we do it beforehand hopefully we'll catch any mistakes before running the robot<br> | |||
We should make a list of all the information Clothos gives us after it solves for the assembly tree. We've had to spent a good chunk of time writing excel formulas and making spreadsheets just so we know exactly how to assemble everything <br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 18:03, 16 June 2010 (EDT)== | |||
Decided to redo all of B along with A. We can do them together on the robot by doubling everything but changing the source plate <br> | |||
Made dilution plate A, and added 60uL of dilution to dilution plate B. 7 with 11 still looked different <br> | |||
Distributed digestion cocktail to both dilution plates<br> | |||
Note: Robot begins pipetting air if it uses the same tip for two long. We stopped the program halfway through to change its tip<br> | |||
Distributed Righty and Lefty parts to reaction plates. Made sure there were duplicate columns for parts being transformed twice<br> | |||
Checked that at this point all wells had the same volume<br> | |||
Put plates in thermocycler to incubate and heat kill<br> | |||
Distributed antibiotics to 10 plates. All of LB agar was contaminated so we will pour that during the rescue step<br> | |||
added ligation mixture to each well and let incubate at room temperature<br> | |||
Began thawing cells (1 tube of each strain)<br> | |||
Poured agar plate<br> | |||
Skipped centrifuge step<br> | |||
Added cells to plate in two different concentrations (30uL, 70 uL)<br> | |||
==[[User:Daniela Mehech|Daniela Mehech]] 12:56, 16 June 2010 (EDT)== | |||
===Robot 2AB assembly Stage 1=== | |||
Yesterday we did stage 1 of our 2AB assembly only on plate B. Today we will repeat the process on plate A without making all of the mistakes that we did yesterday. <br> | |||
<br> | |||
June 15:<br> | |||
Autoclaved our strips for plating. Learned how to work the autoclave machine <br> | |||
<br> | |||
Made a new plate layout for the reaction plate. Wells are organized by what strain the part needs to be transformed into to <br> | |||
MISTAKE: Did not listen to Tim and organized wells by column instead of rows. Rows are better because the plating strips have 2 rows and 12 columns <br> | |||
MISTAKE: Did not read procedure all the way through in detail and assumed that we could use a well for two transformations. Our dilution and reaction plate needs to have duplicates of the parts that go into both Righty and Lefty strains. We have to add the duplicates in either the dilution or reaction plate <br> | |||
Made the dilution plate B (30ul of DNA into each well + 30ul of NEB2 stock diluted by a factor of 5). Dilution plate had the same layout as methylated stock plate so we did it by hand. Next time, we should consider adding duplicates of the parts being transformed twice.<br> | |||
NOTE: 7 with 11 was frozen in our methylated stocks plate. This is the part that was mini-prepped separately the day before. All other wells weren't frozen. <br> | |||
<br> | |||
Had the robot make reaction plate (distribute digestion mastermix to all wells, distribute all Lefty parts, mix, distribute all righty parts, mix)<br> | |||
While the reaction plate incubated in the black thermocycler (which also did heat kill of the digestion enzymes) we distributed the antibiotics to the 24-well strips. We made antibiotics (dilute stock by 10, add 1ul of dye). This part went fine except we should have labeled the plates before we put them on the robot. Added LB agar to the plates and let them dry next to the flame. <br> | |||
MISTAKE: forgot to make second plate for parts being transformed twice. Column 5 in reaction plate needed to go to two different plates. We made the extra plate by hand<br> | |||
<br> | |||
Had robot add the ligation cocktail to the wells and let it incubate at room temperature. We began thawing our bacteria. <br> | |||
Did transformation of bacteria. We had enough volume to seed at least 60uL of bacteria per column.<br> | |||
MISTAKE: Did not realize that the protocol was for seeding 10uL of bacteria. We wanted to seed about 150uL. We should not have done the centrifugation and aspiration of the supernatant step.<br> | |||
MISTAKE: ejecting tips from multi-channel before releasing cells on plate. We lost 60uL of our cells from column 7.<br> | |||
MISTAKE: We split the volume in column 5 into column 9 (because column 5 had to be transformed into both righty and lefty strains). Thus we also did not have enough volume for those parts. <br> | |||
MISTAKE: not taking a short break after lunch to re-energize and prevent making silly mistakes<br> | |||
===To Do Today=== | |||
*Run plate A and B together | |||
*autoclave more 24-well strips | |||
*Make dilution plate A and add volume to dilution plate B | |||
*arrange reaction plate by rows instead of columns (have each row be transformed by the same strain) | |||
*make more colored antibiotics | |||
*label 24-well strip plates before putting them on robot and don't forget to make two plates for the parts being transformed twice | |||
*do not centrifuge and aspirate supernatant after the recovery step | |||
==[[User:Daniela Mehech|Daniela Mehech]] 20:37, 14 June 2010 (EDT)== | |||
Tim and Shelly picked colonies and mini-prepped for us over the weekend (Thank you!). We mini-prepped one colony (7 with 11), because nothing grew from our initial transformation. We also did RE mapping of 4 mini-preps to confirm that their tubes were labeled correctly (which they were). | |||
[[Image:BgXh mapping stage 0 June 14.JPG]] | |||
{| {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Lane #''' | |||
| align="center" style="background:#f0f0f0;"|'''ID''' | |||
| align="center" style="background:#f0f0f0;"|'''Name''' | |||
| align="center" style="background:#f0f0f0;"|'''Expected fragments''' | |||
| align="center" style="background:#f0f0f0;"|'''''' | |||
|- | |||
| 1||4 with 10 #1||pMLL4-AC+B10sbb43 ||850||1990 | |||
|- | |||
| 2||4 with 10 #2||pMLL4-AC+B10sbb43 ||850||1990 | |||
|- | |||
| 3||4 with 18 #1||pMLL4-AC+B10sbb13 ||850||2160 | |||
|- | |||
| 4||4 with 18 #2||pMLL4-AC+B10sbb13 ||850||2160 | |||
|- | |||
| 5||4 with 9 #1||pMLL4-AC+B10Sbb20||850||2450 | |||
|- | |||
| 6||4 with 9 #2||pMLL4-AC+B10Sbb20||850||2450 | |||
|- | |||
| 7||9 with 3 #1||pMLL9-CA+Bjh1853||300||1500 | |||
|- | |||
| 8||9 with 3 #2||pMLL9-CA+Bjh1853||300||1500 | |||
|- | |||
| | |||
|} | |||
Christoph and I began planning our Stage 1 assembly by writing a CSV file. We worked on replicating the spreadsheet Jennifer showed us to keep a detailed track of all of our parts. | |||
===To Do=== | |||
Write final CSV file (both for making plates and for the digestion/ligation reaction plate) | |||
Make plates with proper antibiotics for plating | |||
Run Stage 1 on robot, transform, and plate | |||
==[[User:Daniela Mehech|Daniela Mehech]] 19:04, 11 June 2010 (EDT)== | |||
Colonies grew from pMLL5+Bca1152 so I ran a colony PCR and inoculated 4 cultures. From colony PCR (band at 250), decided to miniprep colonies C and D. Sent D to sequencing, added it to parts plate (source) and transformation plate (destination). Mini prep from C went to Working Box<br> | |||
[[Image:Colony PCR Bca1152.JPG|300px]]<br> | |||
Added grown up Lefty and Righty strains to 500ml each of LB. Tim made competent cell stock for us | |||
Finished with Christoph our CVS file for robot to dilute our parts in a new plate. (Note: when we were taking off the lids of the source plate, a few drops sprayed out. We dried the surface with a kimwipe, hopefully nothing got contaminated). Actually did the transformation by hand, but next time we should use the robot. Plated everything. Someone will move the plates from the incubator to the fridge tomorrow | |||
===To Do=== | |||
Check that colonies grew in all of our transformation | |||
Get going on stage 1 of assembly | |||
==[[User:Daniela Mehech|Daniela Mehech]] 14:38, 10 June 2010 (EDT)== | ==[[User:Daniela Mehech|Daniela Mehech]] 14:38, 10 June 2010 (EDT)== | ||
Sequences for all parts we made were good (pMLL4+Bjh1882, pMLL5+Bca1091, pMLL6+Bjh2245)<br> | Sequences for all parts we made were good (pMLL4+Bjh1882, pMLL5+Bca1091, pMLL6+Bjh2245)<br> | ||
Latest revision as of 15:29, 13 August 2010
Daniela Mehech 18:29, 13 August 2010 (EDT)
Daniela Mehech 21:17, 12 August 2010 (EDT)
Tuesday (8/10):
Transformed all goal parts.
Paused assembly on 4 remaining parts
Wednesday (8/11):
Picked 2 colonies for all goal parts
did not pick colonies for Igem 107 in CA
Thursday (8/12):
Mini-prepped all goal parts except 4
Picked colonies for B3 in plates A and B, and C3 and D3 in plate A
To Do Friday:
Mini-prep those 4 parts
RE map everything
Daniela Mehech 17:34, 9 August 2010 (EDT)
Friday (8/6):
Threw out 107, 109, 115, 116 because we plated them on the wrong antibiotics. Need to redo their assembly
mini-prep, RE, sequence 130, 135
Add terminator to 112, 113
Saturday (8/7):
pick colonies for 107, 109, 112, 113, 115, 116, 126
Sunday (8/8):
mini-prep 107, 109, 112, 113, 115, 116, 126
Monday (8/9):
Assemble 128, 129
Transfer 107 to CA (in order to assemble 127)
Plate 140 on arabinose
Transform 112, 113, 115, 116 into lefties (we were using righty methylated minipreps when trying to add the terminator)
Daniela Mehech 19:25, 5 August 2010 (EDT)
To Do Thursday:
Analyze sequencing data
Mini-prep, RE, sequence 124, 125, 134, E/B transfer
Transform and plate 125, 126
Assemble and transform GFP with ig114
Redo 107, 109, 112, 113, 115, 116, 130, 135
Daniela Mehech 19:33, 4 August 2010 (EDT)
Mini-prep and Map Catch ups
send in sequencing 133, 136, 138
pick colonies 124, 134, E/B transfer for ig114
Manual Assembly 125, 126
Add terminator 107, 109, 115, 116
regular assembly 130, 135, 112, 113
Daniela Mehech 17:15, 3 August 2010 (EDT)
August 2nd:
Most of Stage 4 parts come out with good sequencing.
Problems:
ig114 has two bp mutations that got passed on to just about every single part. The mutations shouldn't affect the function but we're going to test that to make sure
b1006! is missing from various parts because of iGEM 66 failure. We're going to add those to the ends of the parts
A couple of parts did not work and we can't figure out why. We'll remake their parent parts and try again
igem111 that was in Laeretes was wrong. We put in a different copy from Gandalf in Laeretes that sequenced correctly. We're re doing those parts
igem 92 was wrong. We're redoing it and those parts
Transformed the parts we need to remake
August 3:
Picked Colonies for parts that transformed
Three parts didn't grow so we made them by hand. Not sure if the bands in the gel came out the right size
We have 11 sequence confirmed goal parts!
Daniela Mehech 19:53, 30 July 2010 (EDT)
July 28:
Mini-prepped and did RE map
RE map came out bad for almost all of Stage 4 parts. For some reason a lot of parts were not being added.
Sent in Stage 2 and Stage 3 parts that had small parent parts (because RE mapping isn't sensitive enough to show us if small parts were assembled correctly)
Picked more colonies from Stage 4
Re-did stage 4 and included some Stage 4 Catchups
July 29:
Mini-prepped Stage 4 Round 1 re-picks
Picked colonies for Stage 4 Round 2
Analyzed sequencing results - apparently iGEM 66 never took on it's righty parent part and everything that came from igem 66 is missing the terminator
July 30:
Mini-prepped Stage 4 round 2
Did RE map of Stage 4 round 1 re-picks and Stage 4 round 2
RE map is still questionable so sent in all of Stage 4 that we had for sequencing
Daniela Mehech 17:55, 27 July 2010 (EDT)
July 26: Did stage 4 assembly and Stage 3 Catchups
July 27: Most things grew except for igem119 and igem104. igem113 sequenced wrong so needs to be redone
Picked colonies for things that grew, but did not start catchups just yet
To Do
Mini-prep everything
Do Stage 3 cathcups (106 and 104) and Stage 4 catch ups that we can do without 106 and 104 (about 6 out of 10)
sequence some goal parts
Do last of stage 4 catchups
Assay!
Daniela Mehech 14:46, 23 July 2010 (EDT)
July 22: Mini-prepped re-do's of stage 1 and what we had for stage 2. RE map came out good (see Christoph's notebook). We made a plate of all good Stage 1 and Stage 2 parts.
Picked colonies for sbb18, igem64 and igem65. These still need to be added to our final plate
Today: Mini-prep and RE map sbb18, igem64 and igem65, and added them to final plate. See RE map below

Did Stage 3 and stage 2 assembly catchups
Daniela Mehech 19:12, 21 July 2010 (EDT)
Most of Stage 2 parts grew although very sparsely. Picked colonies for them, will mini-prep tomorrow morning. We will re-do the 4 missing parts when we get RE map and co-transformation plate results, that way we only have one catch-up group
Picked colonies for stage 1 re-transformations. They all grew except for iGEM65 because we used DNA from the wrong well. Transformed iGEM65 again using DNA from Rosencratz
iGEM64 did not grow with stage 2. Doing assembly by hand (separate digests, gel purified, zymo column). We're re-transforming sbb18 in AK from Mad Hatter to try to get a better mini-prep. If manual assembly still doesn't work we'll have to try again with new sbb18 mini-preps
To Do
Mini-prep stage 2 and stage 1 parts. RE map.
Make new plates for final stage 1 and 2 parts.
Do Stage 2 catch-ups
Pick colonies for 65,64 and sbb18
Daniela Mehech 14:48, 20 July 2010 (EDT)
Christoph picked colonies, mini-prepped, ran RE on Stage 1 parts and did the catchups. Check his page for gel pictures. We have all the stage 1 parts except for one where we used the wrong parent part twice. We did Stage 2 on yesterday. Today none of the rigthies grew and 5 of the 6 Lefties grew.
possible sources of error:
-put antibiotics in wrong location on robot
-Ophelia layout is wrong
-DNA was too dilute
-Cells were too dilute
to do:
-check Ophelia's layout
-col PCR on things that did grow
-Re do stage 2 with higher DNA and Cell concentration
Colony PCR of what did grow from Stage 2 Round 1:

Daniela Mehech 23:27, 12 July 2010 (EDT)
July 10: Broke my back; will return to lab later this week. Christoph doing project solo for now
July 9: Transformed cells and plated them
Daniela Mehech 16:46, 8 July 2010 (EDT)
July 7: Picked 4 colonies for each part. Re-made 7 parts that did not grow well/at all
Sent 7 with 10 to sequencing
Today:
66% of parts had all 4 colonies co-transformed. We've gone back to troubleshooting
7 with 10 sequenced perfectly, but we've left them in the tubes instead of moving them to the plate
Troubleshooting:
Doing a RE digest to see if parts are methylated correctly (these are all lefties):

Another RE digest:

Triple Digest

We began assembly again, this time with more dilute DNA to see if this helps reduce co-transformation. We did digestion and left the ligation over night
Daniela Mehech 21:29, 6 July 2010 (EDT)
RE mapping of 3 parts that we repicked (6 with 24 in AK, KA and 7 with 24). They all came out faint, but most look good)

Did stage 1 assembly without a problem.
Picked Colonies for 7 with 10. Colony PCR results shown below; they all look good
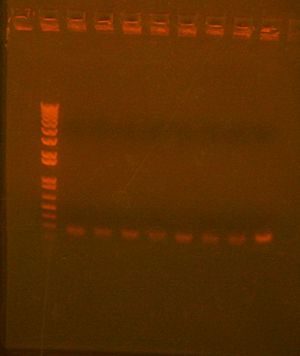
To Do
Check colony PCR result for 7 with 10, mini-prep pick colonies, colony PCR, streak and inoculate for stage 1 assembly
Daniela Mehech 16:42, 5 July 2010 (EDT)
Re-digested parts that showed up faint or not digested:

Everything came out good the second time with the exception of 7 with 10. We noticed that we've been using a mini-prep of 7 with 10 that had a bad sequencing result. We did a lefty transformation of the 7 with 10 that mini-prepped correctly
We chose to repick colonies from 4 parts that did not show up correctly on the RE map at all.
To Do
Pick colonies from 7 with 10 Mini-prep new colonies
Daniela Mehech 15:32, 2 July 2010 (EDT)
Only one colony from the manual assembly (out of 32) was not co-transformed (the same one that had the correct band in the colony PCR gel)
RE map of Methylated Parts for Stage 0:
To Do
Decide from RE map gel which parts need to be re-digested, re-picked or re-transformed
Daniela Mehech 17:27, 1 July 2010 (EDT)
Colony PCR of igem10_064 when Christoph and I made it by hand. Only one lane has a band at 1200. We mini-prepped all of them using the 8-strip method.
We picked colonies for all of our new methylated strains. Except for B4, nothing grew on that so we transformed
To Do
Mini-prep methylated parts RE map and analyze gel. Make plate with only correct parts that have been confirmed by RE mapping Pick colonies for B4, mini-prep at end of the day Check for co-transformation
Daniela Mehech 17:48, 30 June 2010 (EDT)
We used the wrong plate layout when we were remaking our parts - none of them work and we had to redo them.
Our co-transformation experiment didn't work (next to nothing grew), so we're changing it and doing it again.
We decided to start over from our unmethylated plate - we transformed every part into Righty/Lefty strains
To Do
pick colonies from re-made parts. Mini-prep at end of the day
pick colonies from co-transformation experiment and streak for triple resistance. Mini-prep at end of the day
Mini-prep the one part that was correct (6 with 23) and RE map it
Daniela Mehech 17:42, 29 June 2010 (EDT)
All 16 colonies of all 3 parts that we picked were co-transformed. No matter how many times we do it, either manually or with the robot we keep getting a high rate of co-transformation. We're going to test transforming with different concentrations of DNA, different amounts of cells, and a higher concentration of enzyme.
We transformed more parts (those that we are still missing and that did not have enough volume in plate B). They should be done by Wednesday, then we can do robot assembly on Thursday. By Thursday then maybe our experiment will we show us what concentration of DNA/cell/enzymes work best to minimize co-transformations
To Do
Check all of experimental parts (igem10_072) for co-transformation (pick colonies and streak)
Pick colonies, mini-prep, RE map and add newly made parts to dilution plate
Daniela Mehech 19:13, 28 June 2010 (EDT)
Began making dilution plate - transferred 20 ul of parts from either plate B or plate A (whichever had better RE map). Some parts though did not have enough volume in plate B and hadn't been RE mapped in plate A. We're just going to re-transform a big patch of parts so we know for sure that they're correct and that we have enough volume.
Our sequences for vectors pmLL8 and pmLL9 were switched, which explains why we thought we had so many parts wrong. Almost all of the parts in plate B are correct but we can't use half of them because we don't have enough volume. We're not using parts from plate A that were not RE mapped either. We're re-transforming parts and re making them. Now that we've determined that for the most part we were using the correct parts we need to keep looking for an explanation of why our stage 1 parts did not come out right.
Tim picked colonies for us from the parts that re-transformed on Friday. We mini-prepped them and RE mapped. Now that we corrected our sequences all the mini-preps came out correct and we added the newly-made parts to our dilution plate.
We picked colonies from the composite parts we're making by hand. At the end of the day we could tell everything was co-transformed except for igem10_076. It only had 5 colonies grow. We mini-prepped all 5 colonies and picked 16 more colonies for the three other parts.
To Do
re-make parts that we did not have enough volume of in plate A and that we just don't have at all (7 with 10)
Check for co-transformation in manual parts. RE map igem10_076 to determine if it is correct
Daniela Mehech 17:54, 25 June 2010 (EDT)
Transformed 7 unmethylated parts into Righty/Lefty strain. Left them in incubator for someone to remove them tomorrow
Did manual assembly of 4 parts (the 4 goal parts in stage 1, but we transformed them into Righty)
Made plate layouts and wrote CSV files in preparation for robotic assembly. We are not going to having any mislabeled wells this time
To Do
Pick colonies and mini-prep (Monday)
Transfer DNA to new stock plate (Monday)
RE Map all of new plate and sequence some parts to ensure that everything is where it should be (Monday/Tuesday)
Repeat Stage 1 Robotic 2A assembly (Tuesday/Wednesday)
Daniela Mehech 18:42, 24 June 2010 (EDT)
Most of plate B mapped correctly. We mapped the parts from plate A that mapped incorrectly on plate B:
To Do
Re-make those parts that mapped incorrectly in both plate A and B (just transform unmethylated parts into Righty or Lefty strain) Do manual 2AB assembly of four parts to determine if the procedure works, at least by hand Make new methylated stock plate (take best parts from plate A, B and the new ones that we make)
Daniela Mehech 16:23, 23 June 2010 (EDT)
We are going to start over with our mehtylated parts so we ran a RE-mapping of our original methylated parts plate to determine if that plate is correct.
Restriction map of methylated parts Plate B:
Part 1:

Part 2:

All the colonies I repicked were co-transformed, so I didn't run the ColPCR gel
RE mapping of random sampling of methylated and unmethylated stocks. Most are right, but a few came out wrong
Daniela Mehech 20:34, 22 June 2010 (EDT)
Mini-prepped selected parts from groups 3 and 4. Some parts were co-transformed/colony PCR showed no good clones. I repicked more colonies for those parts. Did a restri
ction map of the mini-prepped parts. Everything came out with 3 bands. Maybe the digestion did not go to completion.
Because we are getting such a high failure rate Christoph and I started looking over our entire procedure to see if something wrong happened at the beginning. A RE-mapping of our methylated parts plate did not perfectly match our plate layout = somewhere something went wrong and we need to start over

The top gel is RE-mapping of randomly selected methylated parts. The bottom gel is RE-mapping of mini-prepped composite parts
To Do
Look at Restriction Mapping results - decide what to re-transform and what to send for sequences and what to deem finished Look over repicked results (run colony PCR and check co-transformation plate) - decide what to mini-prep and what to retransform RE-map unmethylated parts to see if at least that is correct
Daniela Mehech 14:19, 21 June 2010 (EDT)
Colony PCR results from Group 2
lane 1,3,5,7 = 5 w/ 35 L
lane 2,4,6,8 = 5 w/ 38
lane 9,11,13,15 = 5 w/ 35 R
lane 10,12,14,16 = 6 w/33 L
lane 17,19,21,23 = 6 w/33 R
lane 18,20,22,24 = 9 w/ 8 L
lane 25, 27,29,31 = 7 w/40
lane 26,28,30,32 = 9 w/ 34 L
lane 33,34,35,36 = 9 w/ 8 R
lane 37,38,39,40 = 9 w/ 34 R
Except for first 8 lanes, everything looked good and we mini-prepped two of each. Mini-prepped lanes 1-8 too and will send them for sequencing
Colony PCR results for group 3:
Loaded as: 1,9,2,10,3,11,4,12,5,13,6,14,7,15,8,16,17,25,18,26,19,27,20,28,21,29,22,30,23,31,24,32
Colony PCR results for group 4:
Restriction Mapping of everything we've mini-prepped (groups 1 and 2)
Right Half
Left Half
To Do
Check for co-transformation for Groups 3 and 4
Mini-Prep good colonies of group 3 and 4
Send to sequencing some parts?
Begin Stage 2 (hopefully all our stage 1 parts are right)
Daniela Mehech 21:28, 18 June 2010 (EDT)
We labeled our transformation plate wrong so when we went to plate the cells many of them were plated on the wrong antibiotics. We still had colonies grow because they were co-transformed and had resistance to all antibiotics. This also explains why so many colonies grew when we tested for co-transformation. We have split our stage 1 parts into 4 groups:
1. Colonies grew on proper antibiotics and were not co-transformed. There are 12 of these. We mini-prepped these today and will do restriction mapping properly on Monday
2. Colonies that grew on wrong antibiotics need to be redone. There are 10 of these.We digested, ligated and transformed them today.
3. Parts that we thought we hadn't done (see yesterday's notes), but it turns out we did do them without realizing it since our plates were labeled wrong. Since we did them twice and they were all plated correctly the second time we will pick colonies, do Colony PCR, check for co-transformation and mini-prep these 9 on Monday.
4. Colonies that grew on the right antibiotics but were co-transformed. We will pick more colonies from the original plate to do ColPCR
Daniela Mehech 18:37, 17 June 2010 (EDT)
We got cell growth for every part we plated (although in some parts there were very few colonies, about 3 or 4).
We made a Colony PCR Mastermix and tried to distribute it to two PCR plates with the robot but the robot kept giving us an error message (It said it couldn't pipette 19uL if there was 17uL in the tube but there was 2 ml in the tube and we wanted it to pipette 17uL). We gave up and I pipetted all 136 wells by hand.
Christoph and I picked colonies, added them to the PCR plate, innoculated them in a 96-well plate and left them in the incubator. We ran a ColPCR3K program on the both PCR plates.
Christoph noticed that we had based all of our csv robot files from the wrong list. We made all of the parts we needed but some of the parts had to be inserted into multiple vector backbones. We began making the 6 forgotten parts by hand. 3 of these parts need to be transformed into both Righties and Lefties so we're making 9 parts by hand.
We dabbed broth from our 96-well inoculated plate on both AKC plates and LB-only plates as negative and positive controls respectively for whether we picked any co-transformed colonies.
We need to run a huge gel when the PCR finishes at 4:40pm. Then we need to analyze the results. Christoph already named all the new composite parts and calculated their sizes.
To Do
We will mini-prep our good colonies tomorrow. Hopefully we'll have at least two good copies for each part so that we can continue with Plates A and B for stage 2.
We can start planning for Stage 2 assembly when we have free time. If we do it beforehand hopefully we'll catch any mistakes before running the robot
We should make a list of all the information Clothos gives us after it solves for the assembly tree. We've had to spent a good chunk of time writing excel formulas and making spreadsheets just so we know exactly how to assemble everything
Daniela Mehech 18:03, 16 June 2010 (EDT)
Decided to redo all of B along with A. We can do them together on the robot by doubling everything but changing the source plate
Made dilution plate A, and added 60uL of dilution to dilution plate B. 7 with 11 still looked different
Distributed digestion cocktail to both dilution plates
Note: Robot begins pipetting air if it uses the same tip for two long. We stopped the program halfway through to change its tip
Distributed Righty and Lefty parts to reaction plates. Made sure there were duplicate columns for parts being transformed twice
Checked that at this point all wells had the same volume
Put plates in thermocycler to incubate and heat kill
Distributed antibiotics to 10 plates. All of LB agar was contaminated so we will pour that during the rescue step
added ligation mixture to each well and let incubate at room temperature
Began thawing cells (1 tube of each strain)
Poured agar plate
Skipped centrifuge step
Added cells to plate in two different concentrations (30uL, 70 uL)
Daniela Mehech 12:56, 16 June 2010 (EDT)
Robot 2AB assembly Stage 1
Yesterday we did stage 1 of our 2AB assembly only on plate B. Today we will repeat the process on plate A without making all of the mistakes that we did yesterday.
June 15:
Autoclaved our strips for plating. Learned how to work the autoclave machine
Made a new plate layout for the reaction plate. Wells are organized by what strain the part needs to be transformed into to
MISTAKE: Did not listen to Tim and organized wells by column instead of rows. Rows are better because the plating strips have 2 rows and 12 columns
MISTAKE: Did not read procedure all the way through in detail and assumed that we could use a well for two transformations. Our dilution and reaction plate needs to have duplicates of the parts that go into both Righty and Lefty strains. We have to add the duplicates in either the dilution or reaction plate
Made the dilution plate B (30ul of DNA into each well + 30ul of NEB2 stock diluted by a factor of 5). Dilution plate had the same layout as methylated stock plate so we did it by hand. Next time, we should consider adding duplicates of the parts being transformed twice.
NOTE: 7 with 11 was frozen in our methylated stocks plate. This is the part that was mini-prepped separately the day before. All other wells weren't frozen.
Had the robot make reaction plate (distribute digestion mastermix to all wells, distribute all Lefty parts, mix, distribute all righty parts, mix)
While the reaction plate incubated in the black thermocycler (which also did heat kill of the digestion enzymes) we distributed the antibiotics to the 24-well strips. We made antibiotics (dilute stock by 10, add 1ul of dye). This part went fine except we should have labeled the plates before we put them on the robot. Added LB agar to the plates and let them dry next to the flame.
MISTAKE: forgot to make second plate for parts being transformed twice. Column 5 in reaction plate needed to go to two different plates. We made the extra plate by hand
Had robot add the ligation cocktail to the wells and let it incubate at room temperature. We began thawing our bacteria.
Did transformation of bacteria. We had enough volume to seed at least 60uL of bacteria per column.
MISTAKE: Did not realize that the protocol was for seeding 10uL of bacteria. We wanted to seed about 150uL. We should not have done the centrifugation and aspiration of the supernatant step.
MISTAKE: ejecting tips from multi-channel before releasing cells on plate. We lost 60uL of our cells from column 7.
MISTAKE: We split the volume in column 5 into column 9 (because column 5 had to be transformed into both righty and lefty strains). Thus we also did not have enough volume for those parts.
MISTAKE: not taking a short break after lunch to re-energize and prevent making silly mistakes
To Do Today
- Run plate A and B together
- autoclave more 24-well strips
- Make dilution plate A and add volume to dilution plate B
- arrange reaction plate by rows instead of columns (have each row be transformed by the same strain)
- make more colored antibiotics
- label 24-well strip plates before putting them on robot and don't forget to make two plates for the parts being transformed twice
- do not centrifuge and aspirate supernatant after the recovery step
Daniela Mehech 20:37, 14 June 2010 (EDT)
Tim and Shelly picked colonies and mini-prepped for us over the weekend (Thank you!). We mini-prepped one colony (7 with 11), because nothing grew from our initial transformation. We also did RE mapping of 4 mini-preps to confirm that their tubes were labeled correctly (which they were).
| Lane # | ID | Name | Expected fragments | ' |
| 1 | 4 with 10 #1 | pMLL4-AC+B10sbb43 | 850 | 1990 |
| 2 | 4 with 10 #2 | pMLL4-AC+B10sbb43 | 850 | 1990 |
| 3 | 4 with 18 #1 | pMLL4-AC+B10sbb13 | 850 | 2160 |
| 4 | 4 with 18 #2 | pMLL4-AC+B10sbb13 | 850 | 2160 |
| 5 | 4 with 9 #1 | pMLL4-AC+B10Sbb20 | 850 | 2450 |
| 6 | 4 with 9 #2 | pMLL4-AC+B10Sbb20 | 850 | 2450 |
| 7 | 9 with 3 #1 | pMLL9-CA+Bjh1853 | 300 | 1500 |
| 8 | 9 with 3 #2 | pMLL9-CA+Bjh1853 | 300 | 1500 |
Christoph and I began planning our Stage 1 assembly by writing a CSV file. We worked on replicating the spreadsheet Jennifer showed us to keep a detailed track of all of our parts.
To Do
Write final CSV file (both for making plates and for the digestion/ligation reaction plate) Make plates with proper antibiotics for plating Run Stage 1 on robot, transform, and plate
Daniela Mehech 19:04, 11 June 2010 (EDT)
Colonies grew from pMLL5+Bca1152 so I ran a colony PCR and inoculated 4 cultures. From colony PCR (band at 250), decided to miniprep colonies C and D. Sent D to sequencing, added it to parts plate (source) and transformation plate (destination). Mini prep from C went to Working Box
Added grown up Lefty and Righty strains to 500ml each of LB. Tim made competent cell stock for us
Finished with Christoph our CVS file for robot to dilute our parts in a new plate. (Note: when we were taking off the lids of the source plate, a few drops sprayed out. We dried the surface with a kimwipe, hopefully nothing got contaminated). Actually did the transformation by hand, but next time we should use the robot. Plated everything. Someone will move the plates from the incubator to the fridge tomorrow
To Do
Check that colonies grew in all of our transformation Get going on stage 1 of assembly
Daniela Mehech 14:38, 10 June 2010 (EDT)
Sequences for all parts we made were good (pMLL4+Bjh1882, pMLL5+Bca1091, pMLL6+Bjh2245)
Our competent cell cultures did not grow up at all - inoculated 2 more cultures of each strain and they appear to be growing well
Christoph and I double checked to confirm we had all the parts we need in the right vector - we're missing pMLL5+B10sbb37/Bca1152)
We took the part from the 140L stock, did a E/B digest, ligated it with pMLL5 digest and transformed it into jtk049 strain
I updated the pictures of the assembly tree
Began planning to do transformations of all our parts into righty and lefty strains
To Do
Pick colonies from Bca1152 transformation for colony PCR. Plate colonies in both CK plate and Spec plate. Nothing should grow in Spec plate
Make competent Pir cells
Transfer parts from working box to PCR plate
Write file for robot to do our transformations (first make dilutions from PCR plate)
When oligos come in - Biobrick upstream region of ef1-alpha
Daniela Mehech 15:33, 9 June 2010 (EDT)
For first Colony PCR of 1882 and 1091: Bca1091 pick lanes 8 and 10 for miniprepping.(Block #1: Colonies from well 1D and 2A)
For second Colony PCR of 1882 and 1091: Bjh1882 pick lanes 1 and 4 for miniprepping. (Block #2: Colonies from well A and D)
Did all 4 mini-preps (stored in working box) and sent in pMLL4-Bjh1882 (D) and pMLL5-Bca1091 (2A) for sequencing
Expected Colony PCR bands:
- pMLL4+bjh1882 = 849bps
- pMLL6+bjh2245 = 265bps
- pMLL5+bca1091 = 228bps
Colony PCR of pMLL6+Bjh2245 should be at about 265bps. Looks good-mini-preped lanes 2 and 3 and sent both for sequencing
Started growing up Lefty and Righty strains. Picked two colonies of each lefty and righty and grew them up in 10ml LB.
Designed three potential oligos for biobricking part Bjh2341CA (igemTen011, igemTen012, and igemTen013)
Bjh2341CA is a potential choano promoter (its a DNA region about 1kb upstream of ef1-alpha, a highly expressed gene)
TO DO
- Finish generating competent cells for "Lefty" and "Righty" strains.
- Analyze sequencing data for pMLL6+Bjh2245, pMLL4+Bjh1882 and pMLL5+Bca1091
Daniela Mehech 18:24, 8 June 2010 (EDT)
Still working with Christoph
Did EB digest of pMLL6 and did gel purification. Did not take picture of gel because it looked right (a band showed up at approximately 2500)
Ligated pMLL6 digestion with Bjh2245 digestion from yesterday and tranformed into bacteria (not methylated strain, Jtk049)
Colonies appeared from our three transformations (Bjh 1882 with pMLL4and 2 samples of Bca1091 with pMLL5)
We ran a colony PCR on the 3 transformations but the results came out ambiguous (see picture below), so we picked 4 more colonies for more colony PCR

Picked more colonies and ran second gel:

To Do
- Miniprep colonies that we pick for pMLL4+Bjh1882 and pMLL5+Bca1091. These are methylated correctly for the robot. Restriction map for confirmation
- Colony PCR pMLL6+Bjh2245 in Jtk049. These are not methylated correctly. Possibly mini-prep if there is enough time
Daniela Mehech 18:58, 7 June 2010 (EDT)
Did the following with Christoph:
Eco/Bam digest of parts: Bjh2245 (LifeACT), Bjh1882 (RFP), and BCA1091 (Ptet)
Gel purified Bjh1882 and Bjh2245
Small fragment zymo cleanup of BCA1091
Couldn't see fragments. Turns out Bjh2245 is 97bp so we should have done a small zymo clean up instead of running it on a gel
Re-cut Bjh2245 and Bjh1882
Did a small zymo clean up of Bjh2245
Did zymo gel purification of Bjh1882 (see image below)
Next we ligated the digested part with the desired vector
Bjh1882+pMLL4 (AC)
Bjh2245+pMLL6 (KA)
Bca1091+pMLL5 (CK)
We don't have pMLL6 in digested form. We'll digest and ligate it tomorrow
We did transformation with Bjh1882+pMLL4 and Bca1091+pMLL5. We transformed them with a Lefty strain of E.Coli (Pir strain, yellow tube) so that the DNA is already methylated
To Do
Digest pMLL6 with Eco/Bam, ligate it with Bjh2245, transform check transformation of Bjh1882 and Bca1091


















