Biomod/2012/Harvard/BioDesign/design: Difference between revisions
Wesley Chen (talk | contribs) No edit summary |
Wesley Chen (talk | contribs) No edit summary |
||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
<br> | <br> | ||
Our final result was a 10 helix x 14 turn L-DNA sheet connected to the center of a 24 helix by 29 turn D-DNA template. In principle, we could detach this L-DNA layer by addition of | Our final result was a 10 helix x 14 turn L-DNA sheet connected to the center of a 24 helix by 29 turn D-DNA template. In principle, we could detach this L-DNA layer by addition of nucleases to digest the template layer and isolate the nuclease-resistant L-DNA layer. However, due to time constraints and the desire to show the templating as clearly as possible, we have imaged our results with the template attached. | ||
The L-DNA layer is formed by formed by 2 unique, tessellating strands which are connected to the template by handles that stick out of the template. These handles are designed into the strands of the template as an extra piece. For design process details, see our [[Biomod/2012/Harvard/BioDesign/L-DNA layer | approach of the L-DNA Layer]] | The L-DNA layer is formed by formed by 2 unique, tessellating strands which are connected to the template by handles that stick out of the template. These handles are designed into the strands of the template as an extra piece. For design process details, see our [[Biomod/2012/Harvard/BioDesign/L-DNA layer | approach of the L-DNA Layer]] | ||
The template was generated from a series of strands for a 24x29 SST canvas using | The template was generated from a series of strands for a 24x29 SST canvas using Motif 1 designed by Bryan Wei et. al in an ongoing project (results not yet published). A strand diagram of "Motif 1" is shown below and more information on the template modifications can be found [[Biomod/2012/Harvard/BioDesign/small_canvas_SST#Basis_of_Design | here]]. | ||
<center>[[Image:Motif 1.png|500px]]</center> | |||
This canvas | This full canvas, about 105nm x 55nm, is a size that is easy to image by AFM, as shown below. | ||
<center>[[Image:Original24x29_LargeCanvas_1_100.jpg|500px]]</center> | |||
<h5>The original 24 helix x 29 turn unmodified large template: well defined, easy to image </h5> | |||
We decided to template the L-DNA on the interior of the template. This decision was made for a couple reasons: to be able to see the height differences with the AFM within every structure and so that the modified structure's rigidity would be enforced by a border of unmodified canvas. (see more [[Biomod/2012/Harvard/BioDesign/large_canvas_SST|details of Large Canvas Approach]]) | We decided to template the L-DNA on the interior of the template. This decision was made for a couple reasons: to be able to see the height differences with the AFM within every structure and so that the modified structure's rigidity would be enforced by a border of unmodified canvas. (see more [[Biomod/2012/Harvard/BioDesign/large_canvas_SST|details of Large Canvas Approach]]) | ||
Given below | Given below are the 375 SST strands (each of 42 base pairs - [http://openwetware.org/wiki/Biomod/2012/Harvard/BioDesign/introduction#Solution here's a refresher]) which form our large template. | ||
[[Image: LgTemplate.png|500px]] | <center>[[Image: LgTemplate.png|500px]]</center> | ||
Our L-DNA was designed to form a central structure, on top of the blue template (represented in green). | |||
<center>[[Image: LgTemplateLDNA.png|500px]]</center> | |||
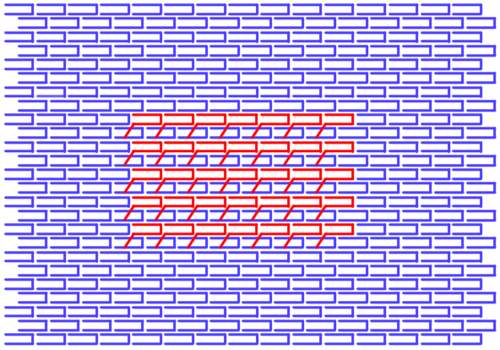
To bind to the L-DNA template, we designed handles coming off of the template at these locations, to grab the complementary handle of one set of L-DNA. | |||
[[Image:LgTemplateHandles.png|500px]] | <center>[[Image:LgTemplateHandles.png|500px]]</center> | ||
The first anneal was with | The first anneal was with the template in the same pot as the first set of L-DNA strands (heated at 90°C down). We also tried forming the structure first and then adding in the first set of ribbons. For other procedures and protocols, refer to [http://openwetware.org/wiki/Biomod/2012/Harvard/BioDesign/protocols this page] | ||
After the first anneal, the second strand | After the first anneal, the second strand was added and then re-annealed but at a lower temperature (40°C)- one that wouldn't melt the strand. We designed the L-DNA layer sequences to have a lower annealling temperature than the template below it (more on the [http://openwetware.org/wiki/Biomod/2012/Harvard/BioDesign/L-DNA_layer L-DNA Layer]. | ||
And our final product, some highlights of our AFM images: | And our final product, some highlights of our AFM images: | ||
[[Image: Ribbon5_LDNA_500nm_final.jpg|500px]] | <center>[[Image: Ribbon5_LDNA_500nm_final.jpg|500px]]</center> | ||
<h5>Note the scale on the right side, the white indicates the highest relative height.</h5> | |||
For more, see [[Biomod/2012/Harvard/BioDesign/large_canvas_SST | More 24x29 Canvas Results]] | For more images, see [[Biomod/2012/Harvard/BioDesign/large_canvas_SST | More 24x29 Canvas Results]] | ||
Other than the AFM to verify our results, we also set up a "sawtooth" gel | Other than using the AFM to verify our results, we also set up a "sawtooth" gel where we juxtaposed the gel shifts of the original template, template with one strand of L-DNA added, and the full templated product. We expected each set of strands that we added to result in a shorter gel shift. And that is indeed what we saw: | ||
{| border = "1" cellspacing = "0" | {| border = "1" cellspacing = "0" | ||
Latest revision as of 23:52, 27 October 2012
<html>
<head>
<link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'>
</head>
<style>
/*BUT ACTUALLY*/ body {
font-family: 'Open Sans', sans-serif; overflow-y: scroll;
}
.container {
background-color: #ffffff; margin-top:0px
} .OWWNBcpCurrentDateFilled { display: none; }
h5 {
font-family: 'Open Sans', sans-serif; font-size: 11px; font-style: normal; text-align: center; margin:0px; padding:0px;
}
- column-content
{
width: 0px; float: left; margin: 0 0 0 0; padding: 0;
} .firstHeading {
display:none; width:0px;
}
- column-one
{
display:none; width:0px; background-color: #ffffff;
}
- globalWrapper
{
width: 900px; background-color: #ffffff; margin-left: auto; margin-right: auto
}
- content
{
margin: 0 0 0 0; align: center; padding: 12px 12px 12px 12px; width: 876px; background-color: #ffffff; border: 0;
}
- bodyContent
{
width: 850px; align: center; background-color: #fffffff;
}
- column-content
{
width: 900px; background-color: #ffffff;
}
- footer
{
position: center; width: 900px;
} @media screen {
body { background: #000000 0 0 no-repeat; /* changed default background */ }
}
- menu
{
position: fixed; float: left; width: 180px; padding: 10px; background-color: #FFFFFF;
}
- pagecontent
{
float: right; width: 620px; margin-left: 300px; min-height: 400px
}
- toc { display: none; }
/*Expanding list*/ ul { list-style: none; }
- exp li ul { display: none; }
- exp li:hover ul { display: block; }
- exp li a:active ul { display: block; }
a:link {color:#FF6060;} a:visited {color:#FF6060;} /* visited link */ a:hover {color:#B24343;} /* mouse over link */ a:active {color:#B24343; } /* selected link */
</style> </html>
Design
Our final result was a 10 helix x 14 turn L-DNA sheet connected to the center of a 24 helix by 29 turn D-DNA template. In principle, we could detach this L-DNA layer by addition of nucleases to digest the template layer and isolate the nuclease-resistant L-DNA layer. However, due to time constraints and the desire to show the templating as clearly as possible, we have imaged our results with the template attached.
The L-DNA layer is formed by formed by 2 unique, tessellating strands which are connected to the template by handles that stick out of the template. These handles are designed into the strands of the template as an extra piece. For design process details, see our approach of the L-DNA Layer
The template was generated from a series of strands for a 24x29 SST canvas using Motif 1 designed by Bryan Wei et. al in an ongoing project (results not yet published). A strand diagram of "Motif 1" is shown below and more information on the template modifications can be found here.

This full canvas, about 105nm x 55nm, is a size that is easy to image by AFM, as shown below.

The original 24 helix x 29 turn unmodified large template: well defined, easy to image
We decided to template the L-DNA on the interior of the template. This decision was made for a couple reasons: to be able to see the height differences with the AFM within every structure and so that the modified structure's rigidity would be enforced by a border of unmodified canvas. (see more details of Large Canvas Approach)
Given below are the 375 SST strands (each of 42 base pairs - here's a refresher) which form our large template.

Our L-DNA was designed to form a central structure, on top of the blue template (represented in green).

To bind to the L-DNA template, we designed handles coming off of the template at these locations, to grab the complementary handle of one set of L-DNA.
The first anneal was with the template in the same pot as the first set of L-DNA strands (heated at 90°C down). We also tried forming the structure first and then adding in the first set of ribbons. For other procedures and protocols, refer to this page
After the first anneal, the second strand was added and then re-annealed but at a lower temperature (40°C)- one that wouldn't melt the strand. We designed the L-DNA layer sequences to have a lower annealling temperature than the template below it (more on the L-DNA Layer.
And our final product, some highlights of our AFM images:

Note the scale on the right side, the white indicates the highest relative height.
For more images, see More 24x29 Canvas Results
Other than using the AFM to verify our results, we also set up a "sawtooth" gel where we juxtaposed the gel shifts of the original template, template with one strand of L-DNA added, and the full templated product. We expected each set of strands that we added to result in a shorter gel shift. And that is indeed what we saw:
| ||||||||||||||||||||

|
Supplement: D-DNA and L-DNA strand sequences