Biomod/2012/UTokyo/UT-Hongo/Assembly: Difference between revisions
No edit summary |
|||
| Line 52: | Line 52: | ||
===Outlook=== | ===Outlook=== | ||
First picture shows that the origami with all staples was formed as designed. Also, these pictures may show that the first origami differ from the second one in the structure between two squares. | First picture shows that the origami with all staples was formed as designed. Also, these pictures may show that the first origami differ from the second one in the structure between two squares. | ||
There was the point where it seemed that origami connected with each other aside. This is probably because each Shell was attracted each other by π-π stacking interaction. We designed Shell as base pair lined lengthways, so it was appropriate for Shell to connect sideways by π-π stacking interaction. | |||
Revision as of 11:11, 19 October 2012
<html> <head> <style> <!-- HIDE WIKI STUFF -->
- column-one { display:none; width:0px;}
.container{background-color: #ffffff; margin-top:0px} .OWWNBcpCurrentDateFilled {display: none;}
- content { width: 0px; margin: 0 auto auto 0; padding: 0em 0em 0em 0em; align: center;}
- column-content {width: 0px; float: left; margin: 0 0 0 0;padding: 0;}
.firstHeading {display:none; width:0px;}
- globalWrapper{width:984px; background-color: #ffffff; margin-left: auto; margin-right: auto}
- column-one {display:none; width:0px;background-color: #ffffff;}
- content{ margin: 0 0 0 0; align: center; padding: 12px; width: 960px;background-color: #ffff; border: 0;}
- bodyContent{ width: 960px; align: center; background-color: #ffffff;}
- column-content{width: 984px;background-color: #ffff;}
- footer{display: none; position: center; width: 960px}
@media screen {
body { background: #ffffff url(http://openwetware.org/images/1/14/Biomod-2012-utokyo-uthongo-wrapper-bg.jpg) repeat; }
}
</style>
<style>
- {
text-shadow: 0px 1px 2px #aaa; }
- bodyContent, #column-content, #content {
background: #ffffff url(http://openwetware.org/images/1/14/Biomod-2012-utokyo-uthongo-wrapper-bg.jpg) repeat; }
- column-content {
border-left: 1px solid #888; border-right: 1px solid #888;
}
- header {
width: 960px; height: 304px; background: url(http://openwetware.org/images/3/3b/Biomod-2012-UTokyo-UT-Hongo_header_test2.png) no-repeat; background-size: 100% auto; margin-bottom: 0px; padding: 0px 0px 0px 0px;
/* border-bottom: 1px solid #DEDEDE; */ }
- menu {
display: block; width: 956px; padding: 0px; background-color: black; border: 2px solid #888; margin-bottom: 15px;
}
ul.menu li a {
display: block;
/* border: 1px solid #474655; */
padding: 8px 10px; text-decoration: none;
/* color: #333; */
color: #fff;
/* width: 121px; */
margin: 0px; text-align: center; font-size: large;
}
ul.menu li a:hover{
color: cyan;
}
ul.menu { /* display: none;
position: absolute; */ height: 40px; margin:0; padding:0; list-style:none; float: center;
}
ul.menu li { /* display:inline;*/
margin:0; padding:6px; float: left; position: relative;
}
ul.menu li:hover ul {
display: block; position: absolute; z-index: 100;
}
ul.submenu {
display: none; list-style: none; background-color: #f0f0f0; border: 1px solid #ccc;
}
ul.submenu li {
float: none; margin: 0px;
}
ul.submenu li a {
text-align: left; margin: 0px; width: 340px; padding: 0 0 0 12px; color: black; text-decoration: none; font-size: large
}
ul.submenu li a:hover {
color: black; text-decoration: underline;
}
.thumb {
border-image: url(http://openwetware.org/images/1/14/Biomod-2012-utokyo-uthongo-wrapper-bg.jpg) repeat;
}
.mytable {
border-image: url(http://openwetware.org/images/1/14/Biomod-2012-utokyo-uthongo-wrapper-bg.jpg) repeat;
}
</style> </head> <body>
<!--<div style="text-align: right"> <p><a href="http://openwetware.org/index.php?title=Template:Biomod/2012/UTokyo/UT-Hongo&action=edit">edit this header</a></p> </div>-->
<div style="height: 60px; margin-bottom: 18px;">
<div style="float: left; width: 250px; padding: 3px; background-color: white;">
<a href="http://biomod.net/"><img src="http://openwetware.org/images/8/82/Biomod2012-logo.png" width="250px" height="50px"></img></a>
</div>
<div style="float: right; width: 240px; padding: 3px; background-color: white;">
<a href="http://www.u-tokyo.ac.jp/en/" target="_blank"><img src="http://www.u-tokyo.ac.jp/en/images/banner/UT-logo.gif" width="234" height="60" border="0" alt="The University of Tokyo"></img></a>
</div>
</div>
<div id="header"> </div>
<div id="menu">
<ul class="menu">
<li class="toppage"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo">Top</a></li> <li class="motives"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Intro">Motives</a></li> <!-- <li class="design"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Function">Design</a></li> --> <li class="result"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Assembly">Design & Results</a> <ul class="submenu"> <li><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Assembly#Assembly_of_the_DNA_Shell">Assembly of the DNA Shell</a></li> <li><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Assembly#Capturing_ability">Capturing Ability</a></li> <li><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Assembly#Immobilizing_on_microfluidic_device">Immobilizing on microfluidic device</a></li> <li><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Assembly#Supporting_Enzyme">Supporting Enzyme</a></li> </ul> </li> <li class="method"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Method">Method</a></li> <li class="futurework"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/FutureWork">Progress & Beyond</a></li> <li class="team"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Team">Team</a></li> <li class="acknowledgement"><a href="/wiki/Biomod/2012/UTokyo/UT-Hongo/Acknowledgement">Acknowledgement</a></li>
</ul> </div>
</body> </html>
In this section, we write about our experiment.
Assembly of the DNA Shell
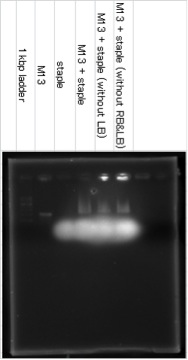
First, we mixed scaffold (used base sequence of M13) and staples, and ascertained whether DNA Shell was hybridized as we had designed by agarose gel electrophoresis and atomic force microscope.
M13 and staples were in 1X TE buffer. We prepared the solution which contain 160nM of each staple DNA and 1.6nM scaffold DNA in 1X TAE/Mg buffer and annealed it from 95℃ to 20℃ in a themal cycler at a rate of 6.25℃ per 10minute 12 steps.
Agarose Gel Electrophoresis
Samples were electrophoreses in a 0.6% agarose gel containing 1xTAE/Mg buffer. The agarose gel was run at 27℃.
The band of M13+staple ran longer than the band of M13, it showed that the structure which had big molecular mass was created. The lower fuzzy band was the band of excess staple DNA. RB and LB means Right Bridge and Left Bridge. These connect three squares of DNA origami.
AFM
These DNA origami (M13 + all staples, M13 + all staples except ones which combine with M13 between the squares (except LB and RB), M13 + all staples except one which combine with M13 between the center square and the right square (except RB)) were observed by using atomic force microscope (AFM) to confirm that these DNA origami were formed correctly. 1xTAE/Mg solution was utilized as buffer.
M13+staple (represent 500nm at one side)



M13+staple (without LB,RB)



Outlook
First picture shows that the origami with all staples was formed as designed. Also, these pictures may show that the first origami differ from the second one in the structure between two squares. There was the point where it seemed that origami connected with each other aside. This is probably because each Shell was attracted each other by π-π stacking interaction. We designed Shell as base pair lined lengthways, so it was appropriate for Shell to connect sideways by π-π stacking interaction.
Capturing ability
Next, we ascertained whether DNA Shell ccaptured target molecules by agarose gel electrophoresis, fluorometry, and AFM.
Agarose gel electrophoresis
Fluorometry
AFM
First, we tried the version of target DNA.
This picture was captured by AFM. In the picture, some square-like structures could be seen. They are exactly DNA Shell. And it is observed that almost central part of the Shell is white. This shows that central part is thicker than the other part of it.
File:Biomod-2012-UTokyo-UT-Hongo-AFMclosecontrol analysis.jpg
In this picture, upper graph display the horizontal height of the line in the bottom picture and each down-pointing triangles are corresponded between upper graph and bottom picture.
以下日本語
DNAに続いてstreptavidinを捕獲できているか確かめました。
このpictureの左上に、2か所白くなっている部分のある四角い構造があるのが確認できます。これがShellです。以下に断面の高低を表した写真を示す。
これらより、以下のことが分かる。
- このShellは横幅91.797nm(約90nm)
- Shell自体の厚さは2.075nm(約2nm)
- Shellの厚くなっている部分の厚さは3.882nmと4.096nm(約4nm)
- Shellの横から21nm付近でほぼ厚さがない部分がある
Shellの構造自体は二重らせんが重なったものなので、厚さ約2nmという結果はorigamiの構造として妥当である。また、厚くなっている部分はほぼ二重らせん2個分の厚さに相当し、Shellどうしが重なっているという事実を裏付けるものである。また、設計段階でのShellの横幅は約120nmであり、今回みられた構造は横幅がその2/3である。Shellの3枚の板のうち2枚が折り重なっていると考えるとこの結果は妥当である。以上のことからSAをShellが捕獲したと推定される。SAは分子量53000Da、大きさ約60Åから、白い部分(厚くなっている部分)がShell本体に比べて小さくなっている。
Immobilizing on microfluidic device
We confirmed if DNA origami is immobilized upon the surface of flowing channel of the microfluidic device.
Control is introduced into microfluidic device
1. A solution containing of fluorescent labeled-DNA origami(M13 + all staples) was introduced into a microchannel on the microfluidic device. After rinsing to remove DNA origami not immobilized on the microchannel, observation of remaining fluorescence from immobilized DNA origami is performed (Fig. 1). The scale bar is 100 μm.
2. The fluorescence was almost kept even if water is introduced into the microchannel (Fig. 2). Decreasing of fluorescent intensity was observed due to destruction of immobilized DNA origami.
3. The fluorescence strength would be weak if the control solution is introduced into the microchannel (Fig. 3).
And we showed the intensity of fluorescence of each iamge to the graph.
Therefore, we could confirme that DNA origami was immobilized onto the surface of the microchannel.
Streptavidin is perfused into microfluidic device
1. A solution containing of fluorescent labeled-DNA origami(attached Biotin) was introduced into a microchannel on the microfluidic device. After rinsing to remove DNA origami not immobilized on the microchannel, observation of remaining fluorescence from immobilized DNA origami is performed (Fig. 4). The scale bar is 100 μm.
2. The fluorescence was kept even if water is introduced into the microchannel (Fig. 5).
3. The fluorescence strength would be weak if the streptavidin is introduced into the microchannel (Fig. 6).
And we showed the intensity of fluorescence of each iamge to the graph.
Supporting enzyme
Based on the results mentioned above, we did further advanced experiment. We ascertained whether DNA Shell supported enzymes.
Background
We used tetramethylbenzidine (TMB), streptavidin with horseradish peroxidase (HRP) labeling and trypsin. TMB can be oxidized for the reduction of hydrogen peroxide to water by peroxidase enzymes such as HRP. When TMB is oxidized, the color of the solution takes on a blue color. If pH of the solution is low (for example, using sulfric acid), the color turns into yellow. The former blue color can be read at a wavelength of nm and the latter yellow color can be read at nm.
We made the use of this chemical reaction. Trypsin is protease. Now suppose the solution with HRP, hydrogen peroxide and TMB. In acid condition, TMB may be oxidized and change color of the solution. However, if trypsin exists with HRP solution, it can be imagined easily that trypsin decompose HRP and the chemical reaction mentioned above does not happen. Then, the DNA Shell is added. The DNA Shell protects HRP by combining streptavidin with biotin sticked to the end of spreading DNA from the Shell, so we can expect the oxidization of TMB and changing color.