Biomod/2013/Aarhus/Materials And Methods/Origami: Difference between revisions
| Line 43: | Line 43: | ||
====Purification with freeze n’ squeeze==== | ====Purification with freeze n’ squeeze==== | ||
The sample was run on a 1 % agarose gel. The band of intereset was cut out and chopped into smaller pieces. The gel pieces were transferred to the filter and placed in a -20˚C freezer for 5 minutes. Subsequently, the filter was centrifuged at 13,000 × g for 15 minutes at room temperature. The agarose gel was retained inside the filter while the purified origamis were isolated in the column. | |||
====Purification with 100 kDA Amicon filters==== | ====Purification with 100 kDA Amicon filters==== | ||
Revision as of 03:59, 25 October 2013
<html> <style> /* ul.menu li.</html>Materials And Methods/Origami<html> a {
color: cyan;
}
- /
- toc {
display: none; }
- mytoc {
background: none; width: 200px; }
.toc { border: 0px solid; }
- toc ul ul,.toc ul ul {
margin: 0 0 0 1em; }
table.toc { background-color: #f0f4f4; }
- mytoc a,#mytoc a:visited {
font-size: normal; color: #222222; }
- mytoc a:hover {
font-color: #009ee0; /* text-decoration: underline; */ }
- wiki-toc {
width: 200px; margin-top: 6px; float: left; }
- wiki-body {
margin-left: 200px; padding-left: 12px; padding-right: 35px; }
- toc #toctitle,.toc #toctitle,#toc .toctitle,.toc .toctitle {
text-align: left; }
- toc h2,.toc h2 {
font-weight: normal; font-size: 17px; }
/*
- wiki-contents A {
color: #00aeef; }
- wiki-contents A:HOVER {
color: #00aeef; }
- /
- toctitle span {
display: none; }
/*
- wiki-body p,#wiki-body li,#wiki-body dd,div.thumbcaption {
font-size: medium; }
- /
/* required to avoid jumping */
- tocScrolWrapper {
/* left: 450px; */ position: absolute; /* margin-left: 35px; width: 280px; */ }
- tocScrol {
position: absolute; top: 0; /* just used to show how to include the margin in the effect */ /*margin-top: 20px; */ /* border-top: 1px solid purple; */ /*padding-top: 19px;*/ }
- tocScrol.fixed {
position: fixed; top: 0; }
- editPageTxt {
text-align: left; padding-left: 15px; }
- editPageTxt P {
clear: both; }
- toPageTop {
float: left; position: relative; top: 18px; left: 13px; color: #d13f31; } </style>
<script type="text/javascript"> $(document).ready(function() { var parentTable = $("#toc").parent(); $('#mytoc').append($("#toc").first());
$('#mytoc').find("#toc").attr("id", ""); parentTable.closest('table').remove(); });
$(document).ready( function() { var top = $('#tocScrol').offset().top - parseFloat($('#tocScrol').css('marginTop').replace( /auto/, 0)); var nav = $('#tocScrol'); var max = $('#indexing').offset().top - nav.height();
$(window).scroll(function(event) { // what the y position of the scroll is var y = $(this).scrollTop();
if (y > top) { // && signs are html decoded thus this construction if (y >= max) { nav.removeClass('fixed'); nav.css({ position : 'absolute', top : max - top }); } else { nav.addClass('fixed'); nav.removeAttr('style'); } } else { nav.removeClass('fixed'); nav.removeAttr('style'); } }); }); </script> <html> <html> <div id="wiki-contents"> <div id="tocScrolWrapper"> <div id="tocScrol"> <div id="wiki-toc"> <a id="toPageTop" href="#">▲</a> <table id="mytoc" class="toc" summary="Contents"> </table> <div id="editPageTxt"> <p> [<a href="http://openwetware.org/index.php?title=Biomod/2013/Aarhus/</html>Materials And Methods/Origami<html>&action=edit">edit this page</a>] </p> </div> </div> </div> </div> <div id="wiki-body"> </html>
Origami
Self-assembly of the plate
Plate assembly was optimized with respect to MgCl2 concentrations, time- and temperature ramps.
Self-assembly buffers (10X) contained 50mM Tris-HCl, 10mM EDTA, 50mM NaCl and MgCl2 in concentrations of 50 mM, 80 nM, 125 mM, 150 mM, 180 mM and 200 mM.
An example of a plate self-assembly reaction with 12.5 mM MgCl2 was prepared by mixing the following:
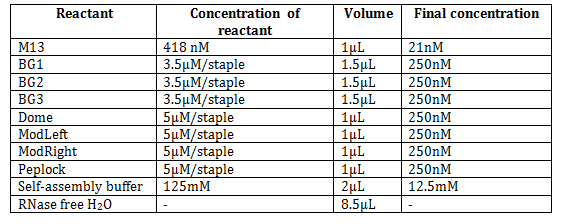
The module ’PepLock’ was replaceable with ’P-Lock’ of the same concentration for the experiments to connect plate and dome.
The samples were incubated in a GS4 PCR machine (G-storm) using a 17 hour non-linear annealing ramp.
17 hour non-linear ramp program
Rapid heating to 95 °C and incubation at 95 °C for 2 minutes
Cycle 1:-1.0 °C/cycle to 65 °C, cycle time = 30 seconds
Cycle 2:-1.0 °C/cycle to 15 °C, cycle time = 20 minutes
After ended program samples are stored at 4 °C
Self-assembly of the dome
An example of a dome self-assembly reaction with 12.5mM MgCl2 was prepared by mixing the following:

The module ’White’ was replaceable with the module ’D-zip’ of the same concentration for the experiments to connect plate and dome, and the module ’Purple’ could be replaced with ’Purple cy5’ of the same concentration.
Characterization
After assembly, the samples were visualized on a 1% agarose gel containing 5mM MgCl2 and 0.5X TBE running buffer, also containing 5mM MgCl2. The gel was pre-stained with SYBR Safe (Invitrogen) and imaged on the Gel Dock (Bio-Rad) or on GE Healthcare Typhoon Trio gel scanning system. Loaded samples were prepared with Orange G with 10% glycerol and a 1kb Plus ladder from Invitrogen was used. Furthermore, AFM and TEM was used to characterize the origami structures.
Purification with freeze n’ squeeze
The sample was run on a 1 % agarose gel. The band of intereset was cut out and chopped into smaller pieces. The gel pieces were transferred to the filter and placed in a -20˚C freezer for 5 minutes. Subsequently, the filter was centrifuged at 13,000 × g for 15 minutes at room temperature. The agarose gel was retained inside the filter while the purified origamis were isolated in the column.
Purification with 100 kDA Amicon filters
The origami plate was purified using 100 kDA Amicon spin filters (Millipore). The filter was washed three times by adding 500 µL of the 1X self-assembly buffer to the filter and centrifuging at 4000 rpm for 8 minutes. The origami sample was added to the column along with the 1X self-assembly buffer to a tatol volume of 500 µL and centrifuged at 4000 rpm for 8 minutes. The column was three times more, before turning the filter upside-down in a fresh collection tube and eluting the sample by centrifuging at 4000 rpm for 2 minutes.
AFM characterization
AFM was done on a mica surface with an AFM (Agilent Technologies), in liquid tapping mode. 1 μL of the sample was pipetted out on the newly cleaved mica and allowed to dry for a minute. The negatively charged origami stick to the negative charged mica through a salt bridge formed by the Mg2+ in the reaction buffer. Then 400μL of the same buffer as the self-assembly reaction proceeded in was added and the sample was mounted in the AFM and imaged with the software, PicoView (Agilent Technologies).
Staining the samples for TEM
TEM grids were lined up by grabbing them by the edge with tweezers and suspending them in the air. The samples were pipetted onto the grids and left for 1 minute to dry. Remaining liquid was removed from the sample by carefully bringing a filter paper in contact with the grid from the side. H2O was added and excess liquid was again removed. The uranylacetate staining solution was added and the grid was left to stain for 1 minute before removing excess stain. The staining procedure was performed twice, and after 24 hours the samples were ready to be imaged using TEM .
Assembly of plate and dome
In preparation for the connection of the dome and plate a larger amount of both plate and dome was made, where the ’White’ module in the dome is replaced with ’D-zip’ and the ’PepLock’ module in the plate was replaced with ’P-Lock’ as follows:
Plate

Dome

1.280 mL dome (1nM) was made in 32 separate 40 µL samples and 120 µL plate (21 nM) in 3 separate samples of 40 µL. The plate was purified with a 100kDa Amicon spin filter. The dome was in separate experiments purified either with a 100kDa Amicon spin filter or using freeze n’ squeeze. During purification of the plate a large excess of 1µM ‘WB-peplock’ (30µL) was added to the filter after the first wash and was left to anneal to the plate for 15 minutes, before unannealed excess of staple strands was washed off. The products of the purification of plate and dome was divided into two samples and mixed with each other. One of the samples was left to anneal at room temperature for 2.5 hours while another was processed in the GS4 PCR machine (G-Storm) at a linear thermal ramp, also for 2.5 hours
Ramp
Rapid heating to 60 °C and incubation at 60 °C for 6 minutes Cycle 1:-1.0 °C/cycle, cycle time = 5 minutes After ended program the samples are stored at 4 °C.
<html></div></div></html> <html> <head> <style>
- indexing {
/* float: left; position: center; */ background-color: #222; border-top: 2px solid #d13f31; color: #006e9c; margin: 0px; padding: 0px 0px 10px 0px; width: 100%; text-align: center; }
.footer-section { padding: 10px; display: table-cell; text-align: left; }
.footer-section-title { font-size: 20px; }
- footer-contents {
color: #006e9c; display: inline-table; }
.footer-section A { color: #006e9c; text-decoration: none; }
.footer-section A:HOVER { color: #00aeef; }
.footer-section ul { list-style-type: square; }
- sitemapTitle {
margin-top: 20px; font-size: 24px; }
- editFooter {
float: right; margin-top: -28px; margin-right: 5px; }
- editFooter A {
color: #006e9c; text-decoration: none; }
.cf:before,.cf:after { content: " "; /* 1 */ display: table; /* 2 */ }
.cf:after { clear: both; }
- bodyContent a[href^="mailto:"], .link-mailto {
background: url() no-repeat scroll right center transparent; padding-right: 0px; color: #006e9c;
}
</style> </head> <body> <div id="indexing"> <div id="sitemap"> <p id="sitemapTitle">SITEMAP | BIOMOD 2013 NANO CREATORS | Aarhus University</p> <div id="footer-contents"> <div class="footer-section"> <p class="footer-section-title">INTRODUCTION</p> <ul> <li><a href="/wiki/Biomod/2013/Aarhus">Home, abstract, animation and video</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Introduction">Introduction</a></li </ul> </div> <div class="footer-section"> <p class="footer-section-title">RESULTS AND DISCUSSION</p> <ul> <li><a href="/wiki/Biomod/2013/Aarhus/Results_And_Discussion/Origami">Origami</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Results_And_Discussion/Peptide_lock">Peptide lock</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Results_And_Discussion/Chemical_Modification">Chemical modification</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Results_And_Discussion/sisiRNA">sisiRNA</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Results_And_Discussion/System_In_Action">System in action</a></li> </ul> </div> <div class="footer-section"> <p class="footer-section-title">MATERIALS AND METHODS</p> <ul> <li><a href="/wiki/Biomod/2013/Aarhus/Materials_And_Methods/Origami">Origami</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Materials_And_Methods/Peptide_lock">Peptide lock</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Materials_And_Methods/Chemical_Modification">Chemical modification</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Materials_And_Methods/sisiRNA">sisiRNA</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Materials_And_Methods/System_In_Action">System in action</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Materials_And_Methods/Methods">Methods</a></li> </ul> </div> <div class="footer-section"> <p class="footer-section-title">SUPPLEMENTARY</p> <ul> <li><a href="/wiki/Biomod/2013/Aarhus/Supplementary/Team_And_Acknowledgments">Team and acknowledgments</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Supplementary/Optimizations">Optimizations</a></li> <li><a href="/wiki/Biomod/2013/Aarhus/Supplementary/Supplementary_Data">Supplementary data</a></li>
<li><a
href="/wiki/Biomod/2013/Aarhus/Supplementary/Supplementary_Informations">Supplementary informations</a> <li><a href="/wiki/Biomod/2013/Aarhus/Supplementary/References">References</a></li> </ul> </div> </div> <div> <p id="copyright">Copyright (C) 2013 | BIOMOD Team Nano Creators @ Aarhus University | Programming by: <a href="mailto:pvskaarup@gmail.com?Subject=BIOMOD 2013:">Peter Vium Skaarup</a>.</p> </div> </div>
<!-- Sponsers --> <div> <img alt="Sigma - Aldrich" src="http://openwetware.org/images/3/39/Sigmaaldrich-logo%28transparant%29.png" width="300px" height="154px"> <img alt="VWR International" src="http://openwetware.org/images/2/28/Vwr_logo.png" width="300px" height="61px"> <img alt="Promega" src="http://openwetware.org/images/7/72/Promega.png" width="175px" height="105px" style="padding-right: 5px; padding-left: 5px;"> <img alt="kem-en-tec" src="http://openwetware.org/images/3/3a/Kementec.png" width="130px" height="129px"> <img alt="Centre For Dna Nanotechnology" src="http://openwetware.org/images/4/4f/CDNA_logo.png" width="420px" height="90px"> <img alt="Dansk Tennis Fond" src="http://openwetware.org/images/9/9a/Dansk_tennis.png" width="250px" height="53px"> </div> <div id="editFooter"> [<a href="http://openwetware.org/index.php?title=Template:Biomod/2013/Aarhus/Nano_Creators/footer&action=edit">edit sitemap</a>] </div> </div> </body> </html>