Biomod/2013/BU/design: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 23: | Line 23: | ||
Past research has shown that a 20 x 30 x 40 nm DNA origami cage with a cavity to be a relatively stable polymer. We aim to use the program caDNAno to first re-validate this design, and then to add functionalization sites to two opposite faces of the cage. Functionalization will take the form of oligonucleotide extensions consisting of poly-T tails, which will later be exploited for peptide attachment to the nanostructure. | Past research has shown that a 20 x 30 x 40 nm DNA origami cage with a cavity to be a relatively stable polymer. We aim to use the program caDNAno to first re-validate this design, and then to add functionalization sites to two opposite faces of the cage. Functionalization will take the form of oligonucleotide extensions consisting of poly-T tails, which will later be exploited for peptide attachment to the nanostructure. | ||
<br /> | <br /> | ||
Figure 1. Displays the original box discovered during the literature search from multiple viewpoints. <br/> | Figure 1. Displays the original box discovered during the literature search from multiple viewpoints. <br/> | ||
| Line 35: | Line 33: | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
<b>Aim 2: To verify the success of the capping protocol and synthesis of the functional polymer-cap.</b><br/> | <b>Aim 2: To verify the success of the capping protocol and synthesis of the functional polymer-cap.</b><br/> | ||
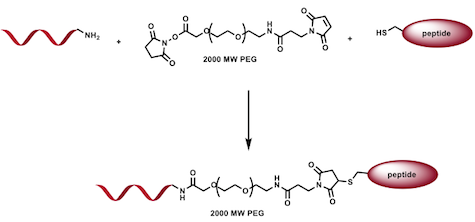
The capping protocol will be verified by synthesizing a fluorescent cap consisting of the oligonucleotide sequence complementary to the functionalization sites as well as a fluorescent tag with an excitation emission spectra significantly different than that used in the agarose gel electrophoresis dye. Two samples, the original literature cage and the modified cage with functionalization sites, will be mixed with the fluorescent cap and incubated. These samples will be run in an agarose gel without dye, imaged, and then soaked in dye and re-imaged. The presence, and lack thereof, of fluorescence in each well will definitively confirm the capping method. Next, the peptide-polymer-cap will be synthesized using the complementary oligonucleotide, a PEG spacing molecule, and the functional peptide. The synthesis will be verified via NMR. | The capping protocol will be verified by synthesizing a fluorescent cap consisting of the oligonucleotide sequence complementary to the functionalization sites as well as a fluorescent tag with an excitation emission spectra significantly different than that used in the agarose gel electrophoresis dye. Two samples, the original literature cage and the modified cage with functionalization sites, will be mixed with the fluorescent cap and incubated. These samples will be run in an agarose gel without dye, imaged, and then soaked in dye and re-imaged. The presence, and lack thereof, of fluorescence in each well will definitively confirm the capping method. Next, the peptide-polymer-cap will be synthesized using the complementary oligonucleotide, a PEG spacing molecule, and the functional peptide. The synthesis will be verified via NMR. | ||
Revision as of 17:14, 26 October 2013
Boston University
BIOMOD 2013 Design Competition
<html>
<style> body { background: #fff; margin: 0; padding: 0; font-family: 'Open Sans', sans-serif; }
- globalWrapper { background: #ffffff; }
- content { background: none; border-style: none; margin: 0; padding: 0; }
- column-one, #contentSub, .firstHeading, #footer { display: none; }
- BU-header { padding: 15px; width: 100%; background: #000000; height: 20px; }
- BU-title { background: #DF0101; padding: 1.8em 0 1.8em 11.5em; line-height: 1.1em; }
- BU-content { min-width: 768px; width: 1000px; margin: 3.5em auto; }
- BU-body { margin-left: 11em; }
- BU-menu { float: left; padding-top: 0; padding-left: 5px; height: 100%; }
.BU-h1, .BU-h4 { color: white; border-bottom: none; padding: 0; margin-top: 14px; margin-bottom: 14px; }
- BU-header a { color: white; font-size: 19px; }
h1 { font-size: 44px; font-weight: 500; border-bottom: none; } h3 { font-size: 27px; font-weight: 300; border-bottom: none; } h4 { font-size: 23px; font-weight: 300; border-bottom: none; } ul.side-nav { display: block; list-style: none outside none; margin: 0; padding: 0; font-size: 14px; line-height: 1.6;} ul.side-nav li.divider { border-top: 1px solid #2383A1; height: 0; padding: 0; } </style> <link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'> </html>
Design Goals
Problem
With an increasing number of unanswered questions in neuroscience, one prominent barrier has been a difficulty in transporting drugs across the blood-brain-barrier. Creating a vehicle capable of delivering drugs to the brain, concentrating the biodistribution within the brain, would be an extremely valuable tool towards advancing treatment for neurological diseases. Additionally, it still is very difficult to study neuronal activity in the brains of monkeys and humans. When working with mice and rats, there exists a wide variety of recording equipment that can be inserted in the brain with ease. If these particles can be delivered to the brain with ease, they can also be packaged with entities that can record or stimulate neurons.
Solution
The goal of our project is to develop a general strategy to functionalize DNA structures with bioactive cues, namely peptides.
We will demonstrate the utility of this approach with two applications. First, we will attempt to get structures into cells in an organized and controlled fashion, and second, get structures to pass through the blood-brain-barrier and enter the brain through the bloodstream.
Our approach can be summarized in three specific aims.
Aim 1: To design and add functionalization sites to a DNA origami nanostructure.
Past research has shown that a 20 x 30 x 40 nm DNA origami cage with a cavity to be a relatively stable polymer. We aim to use the program caDNAno to first re-validate this design, and then to add functionalization sites to two opposite faces of the cage. Functionalization will take the form of oligonucleotide extensions consisting of poly-T tails, which will later be exploited for peptide attachment to the nanostructure.
Figure 1. Displays the original box discovered during the literature search from multiple viewpoints.

Figure 2. Models the creation of the single-stranded functionalization sites in caDNAno

Aim 2: To verify the success of the capping protocol and synthesis of the functional polymer-cap.
The capping protocol will be verified by synthesizing a fluorescent cap consisting of the oligonucleotide sequence complementary to the functionalization sites as well as a fluorescent tag with an excitation emission spectra significantly different than that used in the agarose gel electrophoresis dye. Two samples, the original literature cage and the modified cage with functionalization sites, will be mixed with the fluorescent cap and incubated. These samples will be run in an agarose gel without dye, imaged, and then soaked in dye and re-imaged. The presence, and lack thereof, of fluorescence in each well will definitively confirm the capping method. Next, the peptide-polymer-cap will be synthesized using the complementary oligonucleotide, a PEG spacing molecule, and the functional peptide. The synthesis will be verified via NMR.
Figure 3. Shows the capping process demonstrated in caDNAno, pairing complementary sequences

Figure 4. Diagrams the composition and synthesis of the polymer cap.
Aim 3: To demonstrate and test the utility of functionalized DNA nanostructures via in vitro neuronal uptake and in vivo blood brain barrier entry.
The aforementioned peptides will first be covalently bound to the functionalization sites on the nanostructure. Testing of cellular uptake (neuronal) will then commence, following which a fluorescent tag will be used to verify the uptake of the added nanostructures. Next, in vivo blood brain barrier entry will be tested in mice models via a tail-vein injection protocol. A final assessment of the success and utility of the general functionalization protocol will then be conducted.