Biomod/2013/BU/introduction: Difference between revisions
No edit summary |
No edit summary |
||
| (26 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<h3>Introduction</h3> | <h3>Introduction</h3> | ||
<br/> | |||
The blood brain barrier (BBB) separates circulating blood from the cerebrospinal fluid of the brain, and has evolved to protect the brain from any toxins that might be present in the bloodstream. The barrier itself is a three-pronged defensive layer comprising of tight junctions around capillary endothelial cells, a thick basement membrane, and astrocytotic endfeet. These layers only allow the selective diffusion of small hydrophobic molecules such as oxygen, carbon-dioxide, and certain hormones. However, in terms of therapy, the blood brain barrier has always been a hurdle for drugs targeting the brain since these drugs do not cross over in adequate amounts. Recently, a family of peptides called Angiopeps has been shown to be effective in crossing the blood-brain barrier via low-density lipoprotein (LDL) receptor-transport. Moreover, these peptides have been used to transport biomaterials across the BBB via protein-receptor-mediated-transcytosis. | |||
<center>[[Image:bloodbrainbarrier.png]]</center> | |||
<br/> | |||
We aim to tackle BBB entry via DNA origami technology, a relatively novel method of using bottom-up fabrication to create complex nanostructures in a single-step reaction. The entire concept of DNA origami is based on the properties of complementary base-pairing. While top-down fabrication methods have been the sole means of creating nanostructures with high levels of complexity for a while, a recently developed algorithm allowed for the creation of arbitrary two-dimensional nanostructures from a single-strand scaffold of DNA. The basic tenants of this algorithm involve approximating the shape by double-helical DNA strands, interlacing the scaffold to form crossovers, binding oligonucleotide staple strands to fortify the structure, and finally, removing the double. Since the development of caDNAno, a program dedicated solely for the construction of DNA nanostructures, groups have been able to design virtually any 3-D origami structure, including bricks, cages, cylinders, etc. | |||
<br/> | |||
<center>[[Image:origami1.png]] [[Image:origami2.png]]</center> | |||
<br/> | |||
In their publication, <i>Encapsulation of Gold Nanoparticles in a DNA Origami Cage</i>, Zhao Zhao, Erica Jacovetty, Yan Liu, and Hao Yan demonstrate how to create a rectangular box with a cavity using the bottom-up self-assembly properties of DNA origami. In this particular study, this structure is used to encapsulate gold nanoparticles. This structure allows the surface of the nanoparticles to be tagged with a a controlled number of unique molecules and to control the orientation and intermolecular distance between the nanoparticles. It is stated that a significant advantage in using DNA origami is the spatial resolution of binding sites, on the order of ~6nm, that can be achieved. This structure exhibits a relatively high folding yield and was also found to be fairly robust, withstanding moderate mechanical strain from large nanoparticles placed in the cavity. | |||
< | |||
[[Image: | |||
[[Image: | |||
<br /> | |||
< | |||
-- | |||
Latest revision as of 12:50, 20 October 2013
Boston University
BIOMOD 2013 Design Competition
<html>
<style> body { background: #fff; margin: 0; padding: 0; font-family: 'Open Sans', sans-serif; }
- globalWrapper { background: #ffffff; }
- content { background: none; border-style: none; margin: 0; padding: 0; }
- column-one, #contentSub, .firstHeading, #footer { display: none; }
- BU-header { padding: 15px; width: 100%; background: #000000; height: 20px; }
- BU-title { background: #DF0101; padding: 1.8em 0 1.8em 11.5em; line-height: 1.1em; }
- BU-content { min-width: 768px; width: 1000px; margin: 3.5em auto; }
- BU-body { margin-left: 11em; }
- BU-menu { float: left; padding-top: 0; padding-left: 5px; height: 100%; }
.BU-h1, .BU-h4 { color: white; border-bottom: none; padding: 0; margin-top: 14px; margin-bottom: 14px; }
- BU-header a { color: white; font-size: 19px; }
h1 { font-size: 44px; font-weight: 500; border-bottom: none; } h3 { font-size: 27px; font-weight: 300; border-bottom: none; } h4 { font-size: 23px; font-weight: 300; border-bottom: none; } ul.side-nav { display: block; list-style: none outside none; margin: 0; padding: 0; font-size: 14px; line-height: 1.6;} ul.side-nav li.divider { border-top: 1px solid #2383A1; height: 0; padding: 0; } </style> <link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'> </html>
Introduction
The blood brain barrier (BBB) separates circulating blood from the cerebrospinal fluid of the brain, and has evolved to protect the brain from any toxins that might be present in the bloodstream. The barrier itself is a three-pronged defensive layer comprising of tight junctions around capillary endothelial cells, a thick basement membrane, and astrocytotic endfeet. These layers only allow the selective diffusion of small hydrophobic molecules such as oxygen, carbon-dioxide, and certain hormones. However, in terms of therapy, the blood brain barrier has always been a hurdle for drugs targeting the brain since these drugs do not cross over in adequate amounts. Recently, a family of peptides called Angiopeps has been shown to be effective in crossing the blood-brain barrier via low-density lipoprotein (LDL) receptor-transport. Moreover, these peptides have been used to transport biomaterials across the BBB via protein-receptor-mediated-transcytosis.

We aim to tackle BBB entry via DNA origami technology, a relatively novel method of using bottom-up fabrication to create complex nanostructures in a single-step reaction. The entire concept of DNA origami is based on the properties of complementary base-pairing. While top-down fabrication methods have been the sole means of creating nanostructures with high levels of complexity for a while, a recently developed algorithm allowed for the creation of arbitrary two-dimensional nanostructures from a single-strand scaffold of DNA. The basic tenants of this algorithm involve approximating the shape by double-helical DNA strands, interlacing the scaffold to form crossovers, binding oligonucleotide staple strands to fortify the structure, and finally, removing the double. Since the development of caDNAno, a program dedicated solely for the construction of DNA nanostructures, groups have been able to design virtually any 3-D origami structure, including bricks, cages, cylinders, etc.
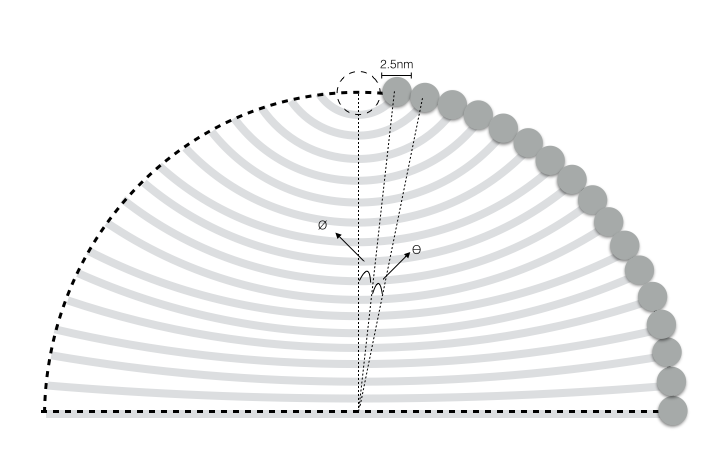

In their publication, Encapsulation of Gold Nanoparticles in a DNA Origami Cage, Zhao Zhao, Erica Jacovetty, Yan Liu, and Hao Yan demonstrate how to create a rectangular box with a cavity using the bottom-up self-assembly properties of DNA origami. In this particular study, this structure is used to encapsulate gold nanoparticles. This structure allows the surface of the nanoparticles to be tagged with a a controlled number of unique molecules and to control the orientation and intermolecular distance between the nanoparticles. It is stated that a significant advantage in using DNA origami is the spatial resolution of binding sites, on the order of ~6nm, that can be achieved. This structure exhibits a relatively high folding yield and was also found to be fairly robust, withstanding moderate mechanical strain from large nanoparticles placed in the cavity.