Biomod/2013/Harvard/introduction: Difference between revisions
| (12 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
=Introduction= | =Introduction= | ||
==Motivation: Detection of Bioagents | __TOC__ | ||
==Motivation: Detection of Bioagents== | |||
[[Image: EasyApplications.png | left | 240 px | easy applications]] | [[Image: EasyApplications.png | left | 240 px | easy applications]] | ||
The importance of being able to detect | The importance of being able to detect bioagents pervades our daily lives as it plays an essential role in biotechnology, medicine, agriculture, and even in military. For example, glucose monitoring for diabetes, fighting bioterrorism, screening for food toxins, and diagnosing a disease all require an efficient method of detecting bioagents. | ||
| Line 14: | Line 16: | ||
[[Image:BiosensorEnzyme.png | frame | center | A Biosensor Enzyme (Adapted from [[Biomod/2013/Harvard/References#General | Mohanty et al, 2006]])]] | [[Image:BiosensorEnzyme.png | frame | center | A Biosensor Enzyme (Adapted from [[Biomod/2013/Harvard/References#General | Mohanty et al, 2006]])]] | ||
Biosensors can be derived from many types of platforms. Perhaps the most well-known biosensor is the commonly used blood glucose monitor. This monitor measures glucose levels by detecting the product of glucose oxidase, hydrogen peroxide, with an electrode. This sensor relies on the natural enzyme glucose oxidase to convert glucose into products that can be easily quantified by electrodes. Such sensors rely on the enzymatic activity of proteins to amplify and convert a signal into forms that can be easily measured. | |||
While there already exists | While there already exists various analytic methods for detection of biogents, such as the glucose monitor and and the more general immunoassays (ELIZA), we seek to harness a particular solution that abounds nature. Because many of biological pathways rely on proteins that accurately detect signaling peptides and transduce the signal further down the pathway, nature already produced many proteins that function as biosensors. At Harvard BioDesign 2013, we sought to take a step in realizing this inspiration from nature. | ||
==Allosteric Switches== | ==Solution from Nature: Allosteric Switches== | ||
Proteins often function as sensors and monitors in nature, and are therefore often used as platforms for engineered biosensors. Many proteins show drastic alterations in conformation or function in response to the presence of another molecule, and thus already act as basic biosensors. These natural responses can be harnessed to create a man-made sensor that is biologically emulative. Standard protein mechanics, such as a change in | Proteins often function as sensors and monitors in nature, and are therefore often used as platforms for engineered biosensors. Many proteins show drastic alterations in conformation or function in response to the presence of another molecule, and thus already act as basic biosensors. These natural responses can be harnessed to create a man-made sensor that is biologically emulative. Standard protein mechanics, such as a change in conformation upon ligand binding, can be modified to occur in response to novel events or conditions. The advantage of using these pre-existing biological system is that there are many, many methods of molecule detection and response already in existence. Natural proteins have evolved to exhibit efficient enzymatic activity and specific binding that would be difficult to engineer <i>de novo</i>. | ||
Natural systems provide excellent starting points for biosensor design, but elements must be altered or substituted to produce novel functionality. In the case of proteins, either the sensitivity and affinity for other molecules, the "input," or the protein's subsequent response to such molecules, the "output," is engineered toward the desired sensor behavior. On the input side, there are thousands of important small molecules, peptides, and larger proteins that are known to cause a conformational change or other detectable response in a protein upon binding. Thus, there are a multitude of proteins capable of sensing molecules already existent in nature. The output response of these proteins is equally varied, but certain enzymatic outputs are particularly useful to researchers. | Natural systems provide excellent starting points for biosensor design, but elements must be altered or substituted to produce novel functionality. In the case of proteins, either the sensitivity and affinity for other molecules, the "input," or the protein's subsequent response to such molecules, the "output," is engineered toward the desired sensor behavior. On the input side, there are thousands of important small molecules, peptides, and larger proteins that are known to cause a conformational change or other detectable response in a protein upon binding. Thus, there are a multitude of proteins capable of sensing molecules already existent in nature. The output response of these proteins is equally varied, but certain enzymatic outputs are particularly useful to researchers. | ||
[[Image:Camconformationalchange.png| frame | left | Protein conformational change after molecule binding]] | [[Image:Camconformationalchange.png| frame | left | Protein conformational change after molecule binding]] | ||
For example, proteins that produce a luminescent or fluorescent output allow the presence of their analyte to be visually observed. Most proteins do not produce a readily observed output though, so they must be augmented or combined to produce a viable sensor. The ideal protein biosensor would be easily modifiable on both the input and output end, allowing easy customization of what the protein senses, and how it reports its sensing. Such a modular protein-based biosensor would have many advantages. First, it would make use of naturally existing activity that can recognize molecules and then deliver an easily detectable signal via an enzymatic response. Secondly, it could be straightforwardly redesigned to sense new compounds and report through a variety mechanisms. Proteins possess a natural ability to detect and respond to other compounds, making them important scaffolds for biosensor development. As our entry to the BioMOD 2013 competition, we worked to demonstrate the power of protein-based biosensors through the creation of a modular sensor. | For example, proteins that produce a luminescent or fluorescent output allow the presence of their analyte to be visually observed. Most proteins do not produce a readily observed output though, so they must be augmented or combined to produce a viable sensor. The ideal protein biosensor would be easily modifiable on both the input and output end, allowing easy customization of what the protein senses, and how it reports its sensing. Such a modular protein-based biosensor would have many advantages. First, it would make use of naturally existing activity that can recognize molecules and then deliver an easily detectable signal via an enzymatic response. Secondly, it could be straightforwardly redesigned to sense new compounds and report through a variety mechanisms. Proteins possess a natural ability to detect and respond to other compounds, making them important scaffolds for biosensor development. As our entry to the BioMOD 2013 competition, we worked to demonstrate the power of protein-based biosensors through the creation of a modular sensor. | ||
==Modular Platform for Allosteric Switches== | |||
Allosteric proteins, proteins that change shape when bound to another molecule, serve as a powerful platform for biosensors. With careful engineering, the ability to change conformation can be harnessed to switch on and off a signal, forming a simple biosensor. By attaching two halves of a transducing enzyme to an allosteric framework, the enzyme's activity can be regulated by the conformational changes of the protein it is bound to, and thus by the concentration of the protein's substrate. Techniques make it possible to vary both the output protein and the substrate of the allosteric framework, in theory allowing modular construction of a biosensor. This goal of modularity, summarized by [[Biomod/2013/Harvard/References#General | Meister & Joshi, 2013]] in the quote below, is the focus of Harvard BioDesign 2013's project. | |||
"Our long-term goal is to develop a modular platform for peptide biosensing in which several input and output domains can be independently optimized using a combination of directed evolution and rational design methods, then combined to create a sensor with the desired input-output functions." | |||
[[Image:ModularPlatform.png | 650 px | left |Modular Platform]] | |||
Our starting point for this project is the BlaCaM protein, which is a fusion of β-lactamase and calmodulin (CaM). To create the fusion, β-lactamase was split into two domains and attached to the C and N termini of calmodulin. CaM exhibits several conformational changes in response to allosteric binding that effect the activity of fused proteins. CaM in its unbound state is in a "closed " conformation, where the C and N termini, are close to each other. The proximity of the two termini means that the two pieces of β-lactamase are also adjacent, resulting in β-lactamase activity. Calcium binding to the calmodulin domain induces a change to the "open" conformation, where the termini are separated. Thus, the two domains of β-lactamase also become separated and activity is lost. Finally, upon binding to certain peptides, the central calmodulin domain of BlaCaM changes to the "bound" conformation and brings back together the two fragments of β-lactamase, activating the β-lactamase protein. Removal of the peptide forces BlaCaM back into the open conformation, and β-lactamase activity is no longer detectable. Through these steps, BlaCaM acts as a protein switch. It turns on detectable β-lactamase activity in the presence of certain peptides, and turns the signal off in the absence of them. The focus of our BioMOD project was to engineer BlaCaM to both switch on new peptides and to produce a new output signal. | |||
[[Image:CaM_Blac.png|frame| The conformational changes and resulting β-lactamase activity of the BlaCaM protein]] | |||
==Project Goals== | |||
Input | |||
# Develop a screening method for directed evolution of BlaCaM | |||
# Engineer new peptide recognition into the BlaCaM protein switch via directed evolution | |||
# Identify important binding residues in the CaM section of BlaCaM by sequencing and analyzing hits from screening | |||
Output | |||
# Modify BlaCaM into GlucCaM and observe a signal from the new protein | |||
# Explore different structure-activity relationships in GlucCaM | |||
# Engineer GLucCaM to output chemiluminescence specifically in response to the target binding conformation changes of CaM. | |||
</div> | |||
Latest revision as of 13:17, 26 October 2013
<html>
<head>
<link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'>
</head>
<style>
body {
font-family: 'Open Sans', sans-serif; overflow-y: scroll;
}
.container {
background-color: #ffffff; margin-top:0px
} .OWWNBcpCurrentDateFilled { display: none; }
h1 {
font-size: 36px; line-height: 36px; padding-top: 5px; border-bottom-width: 0;
}
h3 {
font-size: 18px;
}
h5
{
font-family: 'Open Sans', sans-serif; font-size: 11px; font-style: normal; text-align: center; margin:0px; padding:0px;
}
- column-content
{
/* Uncomment to Dewikify width: 0px; float: left; */ margin: 0 0 0 0; padding: 0;
} .firstHeading {
display:none; width:0px;
}
- column-one
{
display:none; width:0px; padding-top: 35px; background-color: #ffffff;
}
- globalWrapper
{
width: 900px; background-color: #ffffff; margin-left: auto; margin-right: auto
}
- content
{
margin-left: 0px; margin-top: 0px; padding-top: 0px; align: center; /*padding: 12px 12px 12px 12px; width: 30%; background-color: #ffffff; border: 0; */
}
- bodyContent
{
width: 850px; align: center; background-color: #fffffff;
}
- column-content
{
width: 900px; background-color: #ffffff;
}
- footer
{
position: center; width: 900px;
} @media screen {
body { background: #000000 0 0 no-repeat; /* changed default background */ }
}
- menu
{
align: left; width: 10em; padding: 0px 10px 10px 10px; background-color: #FFFFFF; float: left;
}
- pagecontent
{
width: 620px; min-height: 400px; float: left; margin-left: 0px;
}
.group:after {
content: ""; display: table; clear: both;
}
.editsection {
/*display: none*/
}
a:link {color:#FF6060;}
a:active {color:#B24343; }
a:hover {color:#B24343; text-decoration: none}
a:visited {color:#FF6060;} /* visited link */
/*Expanding list*/
- exp { list-style: none; }
- exp li {
height: 1.8em; border-top: 1px solid #dedede; margin: 0 0 0 0; padding-top: .2em
}
- exp li:hover { background-color: #F8F8F8}
- exp li a:hover { display: block }
</style> </html>
Introduction
Motivation: Detection of Bioagents

The importance of being able to detect bioagents pervades our daily lives as it plays an essential role in biotechnology, medicine, agriculture, and even in military. For example, glucose monitoring for diabetes, fighting bioterrorism, screening for food toxins, and diagnosing a disease all require an efficient method of detecting bioagents.
One approach to the problem is the use of biosensors. Biosensors are biologically derived chemical sensing device that recognizes a presence of a certain molecule and outputs a measurable signal in response. It is composed of two parts: the bio-element that recognizes a specific analyte, or bioagent, and the transducer that converts the recognition into a readily detectable output signal.

Biosensors can be derived from many types of platforms. Perhaps the most well-known biosensor is the commonly used blood glucose monitor. This monitor measures glucose levels by detecting the product of glucose oxidase, hydrogen peroxide, with an electrode. This sensor relies on the natural enzyme glucose oxidase to convert glucose into products that can be easily quantified by electrodes. Such sensors rely on the enzymatic activity of proteins to amplify and convert a signal into forms that can be easily measured.
While there already exists various analytic methods for detection of biogents, such as the glucose monitor and and the more general immunoassays (ELIZA), we seek to harness a particular solution that abounds nature. Because many of biological pathways rely on proteins that accurately detect signaling peptides and transduce the signal further down the pathway, nature already produced many proteins that function as biosensors. At Harvard BioDesign 2013, we sought to take a step in realizing this inspiration from nature.
Solution from Nature: Allosteric Switches
Proteins often function as sensors and monitors in nature, and are therefore often used as platforms for engineered biosensors. Many proteins show drastic alterations in conformation or function in response to the presence of another molecule, and thus already act as basic biosensors. These natural responses can be harnessed to create a man-made sensor that is biologically emulative. Standard protein mechanics, such as a change in conformation upon ligand binding, can be modified to occur in response to novel events or conditions. The advantage of using these pre-existing biological system is that there are many, many methods of molecule detection and response already in existence. Natural proteins have evolved to exhibit efficient enzymatic activity and specific binding that would be difficult to engineer de novo. Natural systems provide excellent starting points for biosensor design, but elements must be altered or substituted to produce novel functionality. In the case of proteins, either the sensitivity and affinity for other molecules, the "input," or the protein's subsequent response to such molecules, the "output," is engineered toward the desired sensor behavior. On the input side, there are thousands of important small molecules, peptides, and larger proteins that are known to cause a conformational change or other detectable response in a protein upon binding. Thus, there are a multitude of proteins capable of sensing molecules already existent in nature. The output response of these proteins is equally varied, but certain enzymatic outputs are particularly useful to researchers.

For example, proteins that produce a luminescent or fluorescent output allow the presence of their analyte to be visually observed. Most proteins do not produce a readily observed output though, so they must be augmented or combined to produce a viable sensor. The ideal protein biosensor would be easily modifiable on both the input and output end, allowing easy customization of what the protein senses, and how it reports its sensing. Such a modular protein-based biosensor would have many advantages. First, it would make use of naturally existing activity that can recognize molecules and then deliver an easily detectable signal via an enzymatic response. Secondly, it could be straightforwardly redesigned to sense new compounds and report through a variety mechanisms. Proteins possess a natural ability to detect and respond to other compounds, making them important scaffolds for biosensor development. As our entry to the BioMOD 2013 competition, we worked to demonstrate the power of protein-based biosensors through the creation of a modular sensor.
Modular Platform for Allosteric Switches
Allosteric proteins, proteins that change shape when bound to another molecule, serve as a powerful platform for biosensors. With careful engineering, the ability to change conformation can be harnessed to switch on and off a signal, forming a simple biosensor. By attaching two halves of a transducing enzyme to an allosteric framework, the enzyme's activity can be regulated by the conformational changes of the protein it is bound to, and thus by the concentration of the protein's substrate. Techniques make it possible to vary both the output protein and the substrate of the allosteric framework, in theory allowing modular construction of a biosensor. This goal of modularity, summarized by Meister & Joshi, 2013 in the quote below, is the focus of Harvard BioDesign 2013's project. "Our long-term goal is to develop a modular platform for peptide biosensing in which several input and output domains can be independently optimized using a combination of directed evolution and rational design methods, then combined to create a sensor with the desired input-output functions."

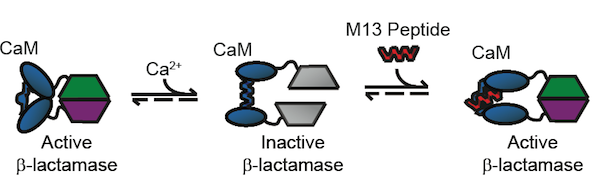
Our starting point for this project is the BlaCaM protein, which is a fusion of β-lactamase and calmodulin (CaM). To create the fusion, β-lactamase was split into two domains and attached to the C and N termini of calmodulin. CaM exhibits several conformational changes in response to allosteric binding that effect the activity of fused proteins. CaM in its unbound state is in a "closed " conformation, where the C and N termini, are close to each other. The proximity of the two termini means that the two pieces of β-lactamase are also adjacent, resulting in β-lactamase activity. Calcium binding to the calmodulin domain induces a change to the "open" conformation, where the termini are separated. Thus, the two domains of β-lactamase also become separated and activity is lost. Finally, upon binding to certain peptides, the central calmodulin domain of BlaCaM changes to the "bound" conformation and brings back together the two fragments of β-lactamase, activating the β-lactamase protein. Removal of the peptide forces BlaCaM back into the open conformation, and β-lactamase activity is no longer detectable. Through these steps, BlaCaM acts as a protein switch. It turns on detectable β-lactamase activity in the presence of certain peptides, and turns the signal off in the absence of them. The focus of our BioMOD project was to engineer BlaCaM to both switch on new peptides and to produce a new output signal.
Project Goals
Input
- Develop a screening method for directed evolution of BlaCaM
- Engineer new peptide recognition into the BlaCaM protein switch via directed evolution
- Identify important binding residues in the CaM section of BlaCaM by sequencing and analyzing hits from screening
Output
- Modify BlaCaM into GlucCaM and observe a signal from the new protein
- Explore different structure-activity relationships in GlucCaM
- Engineer GLucCaM to output chemiluminescence specifically in response to the target binding conformation changes of CaM.

