Biomod/2013/StJohns/results: Difference between revisions
| Line 52: | Line 52: | ||
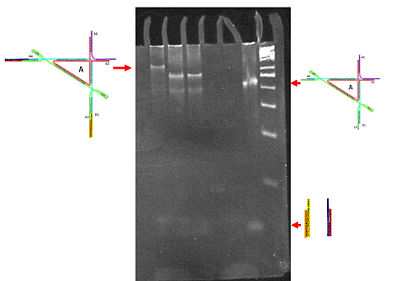
In examining these structures via gel electrophoresis, we would expect that the 90 degree origami triangle with both of its duplexes (or extenders) would run at a slower mobility than the triangle by itself or with one pair of duplexes. It became clear that this sample of triangle with both duplexes (which is the same sample that was used to generate the AFM image above) was a completely different specimen as compared to the other DNA complexes. We utilized a 50 base pair DNA marker to compare the different sizes of the complexes. What is most exciting is that we obtained a sharp and tight band meaning that our sample was one that had a specific DNA complex configuration. This was further supported in our generated AFM images. In future experiments, we could attempt to run a gel similar to this one at either a lower concentration or higher temperatures to eliminate the accumulation of oligomers in the wells. | In examining these structures via gel electrophoresis, we would expect that the 90 degree origami triangle with both of its duplexes (or extenders) would run at a slower mobility than the triangle by itself or with one pair of duplexes. It became clear that this sample of triangle with both duplexes (which is the same sample that was used to generate the AFM image above) was a completely different specimen as compared to the other DNA complexes. We utilized a 50 base pair DNA marker to compare the different sizes of the complexes. What is most exciting is that we obtained a sharp and tight band meaning that our sample was one that had a specific DNA complex configuration. This was further supported in our generated AFM images. In future experiments, we could attempt to run a gel similar to this one at either a lower concentration or higher temperatures to eliminate the accumulation of oligomers in the wells. | ||
[[Image:Lukemanlab-2013-0010. | [[Image:Lukemanlab-2013-0010.jpg|frameless|400px|center]] | ||
Revision as of 08:51, 26 October 2013
<html>
<head>
<link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'>
</head>
<style>
body {
font-family: 'Trebuchet MS', 'Open Sans', sans-serif; overflow-y: auto;
}
.container {
background-color: #ffffff; margin-top:0px
} .OWWNBcpCurrentDateFilled { display: none; }
h5 {
font-family: 'Trebuchet MS', 'Open Sans', sans-serif; font-size: 11px; font-style: normal; text-align: center; margin:0px; padding:0px;
}
- column-content
{
width: 0px; float: left; margin: 0 0 0 0; padding: 0;
} .firstHeading {
display:none; width:0px;
}
- column-one
{
display:none; width:0px; background-color: #ffffff;
}
- globalWrapper
{
width: 1024px; background-color: #ffffff; margin-left: auto; margin-right: auto
}
- content
{
margin: 0 0 0 0; align: center; padding: 12px 12px 12px 12px; width: 1000px; background-color: #ffffff; border: 0;
}
- bodyContent
{
width: 974px; align: justify; background-color: #fffffff;
}
- column-content
{
width: 1024px; background-color: #ffffff;
}
- footer
{
position: absolute; bottom: 0; width: 1024px;
}
- menu
{
position: fixed; float: left; width: 180px; padding: 10px; background-color: #FFFFFF;
}
- resolutionwarning
{
display: none; text-align: center;
}
- pagecontent
{
text-align: justify; float: right; width: 744px; margin-left: 300px; min-height: 400px
}
- toc { display: none; }
@media all {
body { background: #ffffff 0 0 no-repeat;}
}
/*Expanding list*/ ul { list-style: none; }
- exp li ul { display: none; }
- exp li:hover ul { display: block; }
- exp li a:active ul { display: block; }
a:link {color:#007788;} /*unvisited link*/ a:visited {color:#007788;} /* visited link */ a:hover {color:#00ccff;} /* mouse over link */ a:active {color:#00ccff;} /* selected link */
</style> </html>
Summary
- We have synthesized versions of the claw with (‘sticky’) and without (‘blunt’) single-stranded binding elements.
- We have synthesized versions of the claw with and without fluorescent tags for FRET analysis.
- We have visualized the above versions of the claw on AFM to show that they take the predicted shape.
- We have shown that the above versions of the claw form tight bands on a gel, indicating a single primary product of the anneal.
- We have verified that FRET-tagged origami can be visualized in a gel, providing easy visual discrimination between differently-tagged versions of the claw.
- We have demonstrated a binding interaction between the functionalized claw and functionalized capsid as well as a lack of interaction between the nonfunctionalized claw and capsid.
- We have not been able to differentiate bound and unbound complexes via DLS.
- We have generated and isolated FAB fragments for use as binding elements in future claw designs.
- We have demonstrated the potential for selecting claws based on their avidity using a chromatography augmented with photocleavable elements.
- We have synthesized and characterized DO triangles with precisely controlled vertex angles
Data
Claw Visualization
Below are AFM images of the claws alone, with and without binding elements. <html><center><table><tbody align="center"><tr><td><img src="http://openwetware.org/images/thumb/6/66/Lukemanlab-Bluntclaw_afm.png/200px-Lukemanlab-Bluntclaw_afm.png"></td><td><img src="http://openwetware.org/images/thumb/9/9f/Lukemanlab-Stickyclaw_afm.png/200px-Lukemanlab-Stickyclaw_afm.png"></td></tr><tr><td>"Blunt" claw</td><td>"Sticky" claw</td></tr></tbody></table></center></html>
Binding Interaction
We used gel electrophoresis to characterize the binding interaction between origami structures and capsids with and without binding elements.

‘sticky’ DO bind sticky capsids strongly;
however, blunt DO binds sticky capsids.
FRET
Below is a gel demonstrating that versions of the claw tagged with different fluorescent elements can be visually distinguished on a gel.

The six bands on the left are 'blunt' claw and the six on the right are 'sticky' claw.
Odd-numbered lanes contain 0.1 pmol claw, while even-numbered lanes contain 0.2 pmol.
DLS
Our DLS measurements confirmed that the origami control structure and the capsid were of different sizes. However, when performing DLS on a mix of nonfunctionalized origami and capsid, we found only a single peak rather than peaks showing two differently-sized objects. Based on data from other experiments, it is unlikely that this represents a binding interaction. We are currently investigating the source of this anomaly.

Selection

Note the presence of only functionalized claw in the post-cleavage solution.
FAB

Triangles
Utilizing atomic force microscopy (AFM), we managed to image 90 degree origami triangles with two groups of ‘extenders’. These ‘extenders’ are duplexes that have been made at precise stoichiometric ratios. The goal of this project was to successfully attach these two duplexes to the sticky ends of these 90 degree triangles. After obtaining several AFM images showing what appeared to be several triangles with duplexes attached to them, this AFM picture shown here demonstrates the best resolution and separation between each respective triangle. The duplexes that attached to the triangles had a complementary sequence to the sticky ends on the triangle, which is what made it possible for attachment. We have had difficulty in achieving a constant 90 degree angle with both of the duplexes attached. However, when measuring the angles of the triangles with both groups of ‘extenders’ attached, most of our angles were precise (around 100 degrees). In future projects, we hope to gather enough images (such as these) to plot the different angles obtained in an attempt to get as close as possible to 90 degrees.

In examining these structures via gel electrophoresis, we would expect that the 90 degree origami triangle with both of its duplexes (or extenders) would run at a slower mobility than the triangle by itself or with one pair of duplexes. It became clear that this sample of triangle with both duplexes (which is the same sample that was used to generate the AFM image above) was a completely different specimen as compared to the other DNA complexes. We utilized a 50 base pair DNA marker to compare the different sizes of the complexes. What is most exciting is that we obtained a sharp and tight band meaning that our sample was one that had a specific DNA complex configuration. This was further supported in our generated AFM images. In future experiments, we could attempt to run a gel similar to this one at either a lower concentration or higher temperatures to eliminate the accumulation of oligomers in the wells.