Biomod/2014/HKBUteam/methodology
<html>
<head>
<link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'>
</head>
<!-- <$WikiLogoOrSpaceName$> --> <table class="WikiLogoTable WikiElement"> <tr> <td><a href="<$WikiSpaceUrl$>"><img src="http://openwetware.org/images/b/b6/Banner3.jpg" ; width="970px" height="HEIGHT" /></a></td> <td class="WikiLogoName"><a href="<$WikiSpaceUrl$>"><span style="color: white; font-size: 2em;"></span></a></td> </tr> </table>
<style>
/*BUT ACTUALLY*/ body {
font-family: 'Open Sans', sans-serif; overflow-y: scroll;
}
.container {
background-color: #0000ff; margin-top:10px
} .OWWNBcpCurrentDateFilled { display: none; }
h5 {
font-family: 'Open Sans', sans-serif; font-size: 12px; font-style: normal; text-align: center; margin:0px; padding:0px;
}
- column-content
{
width: 5px; float: left; margin: 0 0 0 0; padding: 0;
} .firstHeading {
display:none; width:0px;
}
- column-one
{
display:none; width:0px; background-color: #ffffff;
}
- globalWrapper
{
width: 1000px; background-color: #ffffff; margin-left: auto; margin-right: auto
}
- content
{
margin: 0 0 0 0; align: center; padding: 12px 12px 12px 12px; width: 976px; background-color: #ffffff; border: 0;
}
- bodyContent
{
width: 950px; align: center; background-color: #ffffff;
}
- column-content
{
width: 1000px; background-color: #ffffff;
}
- footer
{
position: center; width: 1000px;
} @media screen { body {
background-image: url("http://www.openwetware.org/images/e/eb/BG12.jpg");
background-repeat: repeat;
background-position: left top;
/* changed default background */ } }
- menu
{
position: fixed; float: left; width: 190px; padding: 5px; font-size: 16px; font-style: normal; text-align: left; margin:0px; padding:0px;
}
- pagecontent
{
float: right; width: 720px; margin-left: 300px; min-height: 500px
}
- toc { display: none; }
/*Expanding list*/ ul { list-style: disc; } ul.a { list-style: none; }
- exp li ul { display: none; }
- exp li:hover ul { display: block; }
- exp li a:active ul { display: block; }
a:link {color:#030303;} a:visited {color:#030303;} /* visited link */ a:hover {color:#10e5e6;} /* mouse over link */ a:active {color:#030303; } /* selected link */
</style> </html>
DNA origami Design
1.DNA origami Structural requirements
DNA origami was designed using caDNAno software (http://cadnano.org/).
2.Design protocol
Oligo list for staple strands and scaffold
All the oligo strands were ordered from TechDragon Limited, Hong Kong.
Note: A 2,3,6 are modified by adding amine on 5’ end.
Recipes for self-assembling of DNA origami
100 mL 20x DNA folding Buffer:
Dilute with RNase free water to 100mL
Staple strands and scaffold are combined in a 10:1 ratio so as to facilitate the chances of correct self-assembly
3.Reviews of the DNA origami design
Conjugation of the load
1. Conjugation of DNA origami with Folic acid, the drug chlorambucil
- Weigh 1.52mg of Chlorambucil and 2.21mg of Folic acid.
- Dissolve Chlorambucil in 25mL Milli-Q water with 1% DMSO. Dissolve folic acid in 25mL Milli-Q water.
- Remove 5uL of Chloramucil solution in a plastic vial A with an autopippette. Remove 5uL of Folic acid solution in a plastic vial B.
- Weigh 1.92mg of EDC and 1.15mg of NHS
- Dissolve EDC and NHS in 5mL of Milli-Q wate separately.
- Remove 5uL of EDC solution and 5uL of NHS solution in Vial A. Remove 5uL of EDC solution and 5uL of NHS solution in Vial B.
- Vortex two vials at a slow speed for 30min.
- Add 10uL of A2 to Vial A. Add 10uL of A3 to Vial B.
- Vortex two vials at a slow speed for 2H, surrounded by ice.
2. Conjugation of DNA origami with graphene quantum dot
Assembly of DNA origami
1. Self-assembly of DNA origami condition
2. DNA origami purification
Agarose Gel Electrophoresis
1.5% agarose gel electrophoresis was conducted at 70 volts for 1 hour 45 minutes in an ice water bath to separate annealed samples.The result of gel electrophoresis was visualized by Bio-Rad ChemiDoc XRS.
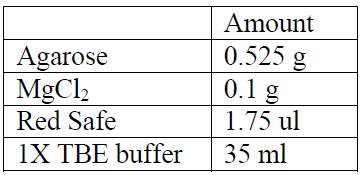
Recipes for a 1.5% Agarose Gel:
Recipes for the Electrophoresis Buffer:

Recipes for Control for Staple & Scaffold Strand:

Gel Extraction using QIAquick Gel Extraction Kit
1. Chop the trimmed gel slice and place the pieces into the filter cup of the Quantum Prep Freeze ‘N Squeeze DNA Gel Extraction Spin Column. Place the filter cup into the dolphin tube.If the volume of your trimmed gel slice is too great to fit into one filter cup, then use two or more and pool the recovered samples at the end of the protocol.
2. Place the Quantum Prep Freeze ‘N Squeeze DNA Gel Extraction Spin Column (filter cup nested within dolphin tube) in a -20° C freezer for 5 minutes.
3. Spin the sample at 13,000 x g for 3 minutes at room temperature.
4. Collect the purified DNA from the collection tube; the agarose debris will be retained within the filter cup of the Quantum Prep Freeze ‘N Squeeze DNA Gel Extraction Spin Column. The DNA is ready to use for PCR, ligations, labeling or other enzymatic reactions. Ethanol precipitation is recommended for applications requiring a more concentrated sample and will also have the effect of further purifying the sample.
References
1. Thuring, R.W.J., Sanders, J.P.M. and Borst, P., Anal. Biochem., 66, 213, (1975).
DNA Precipitation
1. Add 1/10 volume of 3M sodium acetate (NaOAc) (pH 5.2) plus 2-3 volumes of cold 100% ethanol (ETOH) to the solution containing DNA.
2. Place the solution at -20°C overnight.
3. Centrifuge the solution at 14,00O RPM for 10-20 minutes at 4°C. Remove the ETOH and rinse the pellet with 70% ETOH.
4. Centrifuge the re-suspended pellet again at 14000 RM for 5 minutes at 4°C. The ETOH is removed and the pellet is air-dried for about 10 min.
5. Re-suspend the pellet for later use.
Characterized DNA origami by atomic force microscope
1. Dilute DNA origami by phosphate buffer
2. Add 5 ul of conjugated DNA origami onto freshly-cleaved mica surface. Dry sufficiently before AFM study.
The samples were tested on AFM platform under the observation of inverted microscopy (Bruker Nano, Santa Barbara, CA). In order to achieve resolution, the samples were applied onto freshly-cleaved 15x15mm mica sheet (Highest Grave V1, Ted Pella, U.S.A.) for immobilization during pretreatment. A sharp AFM tip (RTESPA, Bruker-nano, Santa Barbara, CA) was used to scan the samples in tapping mode with a scan rate of 1 Hz. All the images were analyzed by NanoScope Analysis Version 1.40.
Confocal microscopy & cytotoxicity assessment
Confocal microscopy for graphene quantum dot DNA origami complex
Cell Subculture for Hep G2 / HK-1
1. Apply aseptic techniques on all apparatus
2. Observe cell
3. Wash the cell with 5 ml PBS
4. Transfer 4 ml PBS to T25
5. Transfer 1 ml trypsin to T25 and, withdraw and discard 3.5 ml of the reaction mixture
6. Incubate the cell in 37oC for 6 minutes
7. Add variable fractions of cDMEM, extract the cell and transfer the cell into a centrifuge tube, top up to 11ml
8. Centrifuge at 700G for 5 minutes
9. Add 10ml cDMEM to a new T25
10. Discard the supernatant and re-suspend the pellet in 1 ml medium
11. Transfer variable drops of the re-suspension to the new T25
12. Incubate the cell in 37oC till next time of subculture
Confocal Microscopy
Day 1
1. Perform cell count
2. Seed cell in 35mm petri dish at a cell density of 1x104 cell/ml
3. Add 2ml medium to petri dish
4. Incubate in 37oC overnight
Day 2
1. Discard medium in petri dish
2. Add 40ul DNA origami to 2 ml medium
3. Add the DNA-origami-containing medium to the 35mm petri dish
4. Incubate in 37oC overnight
Day 3
1. Wash the cell with 1ml PBS for 3 times
2. Add 1ml PBS to the petri dish
3. Observe cell under confocal microscope, using 405nm as the excitation wavelength, 460-560 as the emission wavelength
Cytotoxicity assessment by MTT assay for DNA origami-Drug efficacy
1. Seed cells in 96-well plate at a cell density of 1x104 cell/ml
2. Incubate in 37oC overnight
3. Add enough amount of PBS and medium to two petri dishes separately
4. Observe cell
5. Discard the medium in 96-well plate
6. Wash cell with 150ul PBS each well for 3 times
7. Add 500ul MTT solution into the medium in the petri dish
8. Add 100ul-MTT-containing medium to each well
9. Cover the 96-well plate with aluminum sheet
10. Incubate in 37oC for 3 hours
11. Add 11ml DMSO to a petri dish
12. Pipette out 70ul medium from each well
13. Add 100ul DMSO to each well
14. Shake the plate in 200RPM for 15 minutes
15. Masure the absorbance within 540 and 690nm using the plate spectrometer









