Biomod/2014/experiment.html: Difference between revisions
No edit summary |
|||
| (117 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
body { | body { | ||
display:block; | display:block; | ||
background-color:# | background-color:#f5f5f5; | ||
} | } | ||
| Line 23: | Line 23: | ||
margin-right:0px; | margin-right:0px; | ||
width:1000px; | width:1000px; | ||
height: | height:360px; | ||
z-index:1; | z-index:1; | ||
box-shadow: 0px 5px 8px rgba(0,0,0,0.4); | |||
overflow: visible; | overflow: visible; | ||
text-align: left; | text-align: left; | ||
background-image: url(http://openwetware.org/images/ | background-image: url(http://openwetware.org/images/3/37/Header_tianjin.png); | ||
} | } | ||
| Line 39: | Line 40: | ||
height:40px; | height:40px; | ||
z-index:2; | z-index:2; | ||
font-family: " | box-shadow: 0px 5px 8px rgba(0,0,0,0.4); | ||
font-family: Georgia, "Times New Roman", Times, serif; | |||
text-align: left; | text-align: left; | ||
background-image: url(http://openwetware.org/images/thumb/7/76/Tianjin_index02.jpg/800px-Tianjin_index02.jpg); | background-image: url(http://openwetware.org/images/thumb/7/76/Tianjin_index02.jpg/800px-Tianjin_index02.jpg); | ||
| Line 76: | Line 78: | ||
margin-bottom:40px; | margin-bottom:40px; | ||
z-index:60; | z-index:60; | ||
font-family: " | font-family:Georgia, "Times New Roman", Times, serif; | ||
text-align: left; | text-align: left; | ||
} | } | ||
| Line 82: | Line 84: | ||
#navDiv > ul > li { | #navDiv > ul > li { | ||
float: left; | float: left; | ||
margin-left: | margin-left: 30px; | ||
position: relative; | position: relative; | ||
} | } | ||
| Line 293: | Line 295: | ||
display:block; | display:block; | ||
left:0px; | left:0px; | ||
width: | width:960px; | ||
box-shadow: 0px 5px 8px rgba(0,0,0,0.4); | |||
z-index:3; | z-index:3; | ||
text-align:left; | text-align:left; | ||
font-family: " | font-family:Georgia, "Times New Roman", Times, serif; | ||
font-size: 18px; | font-size: 18px; | ||
color: #000; | color: #000; | ||
background-color: # | background-color: #ffffff; | ||
padding: 20px; | padding: 20px; | ||
} | } | ||
/*hidden section*/ | |||
.firstHeading{display:none;} | |||
#sidebar-main{display:none;} | |||
#p-cactions{display:none;} | |||
#p-personal{display:none;} | |||
</style> | </style> | ||
| Line 310: | Line 320: | ||
<body class="ys01";> | <body class="ys01";background-image: urlhttp://openwetware.org/images/0/0a/Paper.jpg);> | ||
<!--header--> | <!--header--> | ||
| Line 331: | Line 341: | ||
<li class='has-sub'><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html"><span>Project</span></a> | <li class='has-sub'><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html"><span>Project</span></a> | ||
<ul> | <ul> | ||
<li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html# | <li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#Three-arm locker"><span>Three-arm Locker</span></a></li> | ||
<li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html# | <li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#Gold nanoparticle based photosensor"><span> Gold Nanoparticle Based Photosensor</span></a></li> | ||
<li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#dna origami"><span>DNA Origami</span></a></li> | <li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#dna origami"><span>DNA Origami</span></a></li> | ||
</ul> | </ul> | ||
| Line 338: | Line 348: | ||
<li class='active'><a href="http://openwetware.org/wiki/Tianjin_protocol"><span>Protocol</span></a> | <li class='active'><a href="http://openwetware.org/wiki/Tianjin_protocol"><span>Protocol</span></a> | ||
</li> | |||
<li class='active'><a href="http://openwetware.org/wiki/Biomod/2014/future.html"><span>Future Work</span></a> | |||
</li> | </li> | ||
| Line 355: | Line 368: | ||
<div id="apDiv10"> | <div id="apDiv10"> | ||
<a name=" | <a name="Three-arm Locker"></html> | ||
== | ==Three-arm locker== | ||
<html></a></html> | <html></a></html> | ||
DNA strand displacement | <big>DNA strand displacement</big> | ||
Strand displacement is the process through which two strands with partial or full complementarity hybridize to each other, displacing one or more pre-hybridized strands in the process. | Strand displacement is the process through which two strands with partial or full complementarity hybridize to each other, displacing one or more pre-hybridized strands in the process<sup>[1]</sup>. The complementary single-stranded domains (referred to as toeholds) can be the initiator of the strand displacement which progresses through a branch migration process that resembles a random walk. By varying the strength (length and sequence composition) of toeholds, the rate of strand-displacement reactions can be quantitatively controlled over a factor of 10<sup>6</sup>. More importantly, this feature allows engineering control over the kinetics of synthetic DNA devices. <br><br> | ||
[[Image: | [[Image:Fig001.jpg|thumb|900px|center|<html><body>Figure 1-1.</body></html>Panel a shows one example of strand displacement reaction. Single-stranded DNA molecule A reacts with multi-stranded DNA complex X to release strand B and complex Y. Panel c shows that the kinetics of strand displacement can be accurately modeled and predicted from the length and sequence of the toehold domain. Copyright (2011) Nature Chemistry Dynamic Dna nanotechnology using strand displacement reactions Ref.[1]<br>]]<br> | ||
[[Image:Fig002.png|thumb|300px|right|<html><body>Figure 1-2.</body></html>Secondary structure of strand A, strand B, strand C and initiator I with 4 nucleotides in each letter-labelled domain.<br>]] | |||
<big>Design</big> | |||
To approach the goals of local release and selective release, we should design the DNA locker which is expected to intelligently control the open and close of our device.<br> <br> | |||
According to Peng’s paper<sup>[2]</sup>, we designed a self-locking three-arm locker, which utilize strand replacement to assemble its secondary structures. However, we also optimize the three-arm structure with the irreversible characteristics of our lock, which means that it will be not re-locked once it breaks by heating.<br><br> | |||
This locker is composed of strand A, strand B, and strand C, acting as the main body of our lock and strand I, which plays the role of initiator.<br><br><br> | |||
[[Image:Fig003.gif|thumb|300px|right]] | |||
First, the locking process is''' isothermal'''. It is capable to lock at constant room temperature with a simple displacement reaction when initiator I is added. The first step will occur with the exposed toehold of hairpin A nucleating with of I and opening the hairpin. The newly exposed single strand of A will nucleating with the exposed toehold of B and open the B hairpin. After that, the same reaction will happened and lead to the open of C hairpin binding to B. Then the final step occurs in which a single-stranded domain (a* of B) initiates a branch migration that displaces the initiator I from A.<br><br> | |||
When it comes to the characteristics of this structure, to get reach to our first goal, the tree-arm structure is supposed to be with high yield to form with the help of single-strand I and minimal leakage of a system containing only A, B and C.<br><br> | |||
And second, the locker should be''' irreversible'''. If we move single-strand I away and heat the locker, the double strands will melt, the locker opens, and A, B, C will return to independent hairpins. However, without I, even if the all the hairpins are annealed together, they cannot form the three-arm junction kinetically.<br><br> | |||
Third, we design the sequences of the three-arm structure with '''diverse melting temperature''' to reach the purpose of selective release which means to open different locks and release different drugs under various circumstances, so that we can make the decision of how much dose of drugs we release and which drugs. | |||
<big>Characterization and results</big> | |||
[[Image:Fig004.png|thumb|300px|right|<html><body>Figure 1-3.</body></html>Results of repeating the three-arm branched junction experiment of Peng’s paper. Line 1 is hairpin A. Line 2 is hairpin A,B,C and I assemble at room temperature. Line 3 is the mixture of hairpin A,B and C at room temperature(leakage). Line 4 is hairpin A,B and C annealed.<br>]] | |||
To realize those purposes of our design, we study Peng's paper and do some changes. Then modify and select the better groups step by step to get the optimum.<br><br> | |||
First, we try to repeat the experiment of Peng’s paper (Fig 1-3), and with the consequence we discover that with its high stabilization, the length of the sequence is a little bit long and the melting temperature is higher than our expect. | |||
<big>Modification</big><br> | |||
We modified the three-arm locker in the following 2 aspects.<br> <br> | |||
First, in order to increase the yield of three-arm junction in the locking process and to increase the amount of independent hairpins in the unlocking process, we decided to change the number of base pairs in each domain to determine the proper lengths of the three arms. We synthesized 29 groups of various length lockers with numbers of base pairs in each domain varied from 3, 4, 5 and 6. Gel electrophoresis showed that the sequence with 4 bps per domain had the highest yield of three-arm junction in the locking process and relatively low leakage. So we reached the conclusion that 4 bps per domain is the proper length for each arm of the locker to suit our design. And we pick up the comparatively best group''' M4-11''' to do further research.<br><br> | |||
Second, we wanted to get a group of lockers which can open in response to different melting temperature to reach the selective release, so we choose to change the length of the complementary sequence to get different melting temperature. And based on the group M4-11, we would further modify one of the domains specifically. Domain a,b and c are beyond our choice because act as toehold, the modification of them will greatly affect the formation rate of our locker. Domain x,y are also excluded because they are in strand A, and modification of them calls for using different initiator I which makes the selective released system more complicated and difficult to form. We finally chose to modify domain z which has the least effect on the whole structure.<br><br> | |||
We synthesized 11 groups of lockers with group M4-11 and changed the number of base pairs in domain z within the range of 4,6,8,10. Finally, we selected out a high-yield gradient of lockers with different z domain of 4,8,10 bps named '''M4-11'''(Group1), '''M4-11-8-04'''(Group2), '''M4-11-10-01'''(Group3). We also heated the lockers in a temperature range from 50℃ to 65℃ and they could open successfully. As the results we show below(Fig 1-4)(Fig 1-5)<br><br> | |||
And to get comparatively accurate melting temperature of Group1,2,3, we also heating in smaller range of temperature(Fig 1-6).<br> | |||
[[Image:Fig005.png|thumb|700px|center|<html><body>Figure 1-4.</body></html>verification of assemble and in irreversible for three-arm structure for Group1,2,3. In each group line 1 is assemble of hairpin A,B,C and I. Line 2 is mixture of hairpin A,B and C(Leakage). Line 3 is annealing of hairpin A,B and C.<br>]] | |||
[[Image:Fig006.png|thumb|700px|center|<html><body>Figure 1-5.</body></html>verification of assemble and in irreversible for three-arm structure for Group1,2,3. In each group line 1 is at room temperature. Line 2 is heating at 50℃. Line 2 is heating at 59.3℃ and line 4 is heating at 65℃.<br>]] | |||
[[Image:Fig007.png|thumb|700px|center|<html><body>Figure 1-6.</body></html>verification of assemble and in irreversible for three-arm structure for Group1,2,3. In each group line 1 is at room temperature. Line 2 is heating at 60℃. Line 2 is heating at 62.5℃ and line 4 is heating at 65℃.<br>]] | |||
Gel electrophoresis confirms:<br><br> | |||
1.The yield of the locker is high with I added.<br> <br> | |||
2.Without I, at constant temperature, the yield of the locker is relatively low,which means leakage is quiet low for these three groups.<br><br> | |||
3.Without I, annealed the hairpins A, B and C, the yield of locker is also low. So when the double strands are heat and separated, it could hardly to pair again without the initiator I.<br><br> | |||
<html><a name="Gold nanoparticle based photosensor"></html> | |||
==Gold Nanoparticle Based Photosensor== | |||
<html></a></html> | |||
[[Image:SsDNA-Au.gif|thumb|800px|center|<html><body>Fig 2-1.</body></html> Lasers trigger the photothermal effect and the local temperature raises above the melting temperature of the DNA duplex, which allows the nonthiolated strand to dissociate into the surrounding medium while its complementary remains attached to the gold nanoparticle.<br>]] | |||
Functionalization of thiolated DNA on gold nanospheres was carried out by following a previous published protocol with some modifications. Briefly, the disulfide bond in the thiol-modified oligonucleotides was reduced to monothiol using TCEP (10mM, 1h) in water to activate the thiolated DNA. The DNA-SH sample was added drop by drop into the gold nanoparticle solution. The solution was then incubated for 16 h at room temperature. Finally, the resulting solution was centrifuged at 1500g for 60 min and then the supernatant was decanted to remove unbound DNA-SH.<br><br> | |||
We use gel electrophoresis to test the DNA-GNPs. The electrophoresis experiments were performed in 2 % agarose gels at 100 V,1 h. We used 5X TBE as running buffer. Gold colloids in this size range have a deep red color and can be easily visualized in the gels. The mobility of particles on the gel depends on two factors: size and charge. The bigger the size, the slower and the higher the charge, the faster particles will migrate. In the case of negatively charged Au particles, the attachment of negatively charged DNA molecules causes in first place an increase of size that can be seen as a retardation of the band of the gel. Upon attachment of DNA, the mobility of the resulting conjugates was always moderately decreased. Therefore, in agreement with previous reports, we assume throughout this manuscript that attachment of DNA to Au nanoparticles in first order increases the effective diameter of the conjugates which can be directly seen in the retardation of the band of the conjugates in gel electrophoresis experiments. The bands of DNA-Au NP complex will move slower than those of pure Au nanoparticles.<br><br> | |||
The bands can be extracted from the gel by cutting out the agarose piece that contains the band and immersing it into 5×TBE buffer solution. After two days, the Au-DNA conjugates could be diffused out of the gel into the buffer. The extraction procedure ensures that all DNA is really attached to the Au particles, since free DNA migrates in a much faster band.<br> <br> | |||
For the purpose of assembling the origami-GNPs complex,i.e. attaching GNPs to DNA origami as illustrated in Figure 1, we use thiol-modified oligonucleotides (short synthetic DNA sequences), which can be loaded onto the surface of GNPs to combine GNPs and DNA. GNPs are fixed onto the DNA origami by linking them to staple strands whose 5’end are modified with lipoic acid. Upon hybridization between DNA tails on DNA origami and single staple strands on GNPs, GNPs are attached to the DNA origami, resulting in the formation of origami-GNPs complex. DNA origami is used for in vivo delivery of chemotherapeutic drugs in our project. Originally, the photothermal property of GNPs was used in biologically relevant studies to destroy cancer cells, while, in our project, it has been harnessed as a means to optically elicit the release of drugs encapsulated in DNA origami. | |||
[[Image:Gold.png.jpg|thumb|717px|center|<html><body>Figure 2-2.</body></html>solution color reflects the appropriate concentration<br>]] | |||
In order to know gold nanoparticles of which concentration can be easily visualized in the gels, we diluted 13nm gold nanoparticle solution with water of different volumes (the volume ratios of gold nanoparticle solution and solution are 1/1,1/10,1/25,1/50,1/100,respectively). And the result showed that when the the volume ratio is at least 1/25, the diluted 13nm gold nanoparticle solution will show a clear red band in gels. | |||
[[Image:Gold2.jpg|thumb|200px|left|<html><body>Figure 2-3.</body></html>gels result<br>]] | |||
[[Image:Gold3.png|thumb|200px|right|<html><body>Figure 2-4.</body></html>the result of the experiment comparing the bands of 5nm and 10nm gold nanoparticles<br>]] | |||
Then we compared the bands of 5nm and 10nm gold nanoparticles. From the figure we could see that the bands of 5nm gold nanoparticles are single while those of 13nm gold nanoparticlse are continous. This is probably because gold nanoparticles are erratic in TBE buffer while we used 5X TBE as the gel running buffer. And our 5 nm gold nanoparticles were purchased from sigma-aldrich and 13 nm gold nanoparticles were synthesized by citrate reduction of HAuCl4. Hence the stability of our 5nm gold nanoparticles may be better than 13nm gold nanoparticles. | |||
<html><a name="dna origami"></html> | |||
==DNA Origami== | |||
<html></a></html> | |||
<big>Design</big><br><br> | |||
We want to design a simple two-dimensional DNA origami rectangle which can be folded by our three-arm DNA locker into a roll. The dimensions of the origami plate are properly selected so the drug stays encapsulated inside it. The final dimensions are the following: | |||
Length: 50 nm | |||
Width: 35 nm | |||
Thickness: 2 nm | |||
[[Image:Jt.jpg|thumb|550px|right|<html><body>Fig 3-1.</body></html> 1% agarose electrophoresis of DNA origami with different folding processes.<br>]] | |||
To enable the origami to fold into a roll, the three arm locker should be combined with it. The staple strands in two corners of the origami plate are extended with the sequences of strand A, and the staple strands in the other two corners are extended with the sequences of strand C. Strand A and strand C comes from our three-arm DNA locker M4-11-6-01(Group 3) and the one with best isothermal and irreversible properties. | |||
We used the cadnano 2 program for our rectangle shape origami design. | |||
The scaffold used in this design is M13mp18 single strand DNA with 7249 nt, from New England Biolabs. The desired structure and dimensions are achieved with the help of two types of staples: | |||
• core: 58 staples give stability to the whole structure. | |||
• edge: 4 staples give stability to the edges and with extending sequences of A or C. | |||
[[Image:PCR0.png|center]] | |||
<big>Folding and characterization</big> | |||
The mixture of scaffold and different staples were subjected to a thermal annealing process composed of 3 steps that allowed the folding. Different initial temperatures varying from 50.0℃ to 65.0℃ in step 2 were tested. Electrophoresis showed that low initial temperatures in step 2 have relatively high origami yield.(Fig.3-1) | |||
[[Image:AFM.jpg|thumb|550px|center|<html><body>Figure 3-2.</body></html>AFM picture of our designed origami.<br>]] | |||
[[Image:Device1.gif|thumb|550px|center|<html><body>Figure 3-3.</body></html>schematic diagram of our device.<br>]] | |||
==References== | |||
[1] Zhang D Y, Seelig G. Dynamic DNA nanotechnology using strand-displacement reactions [J]. Nature chemistry, 2011, 3(2): 103-113.<br><br> | |||
[2] Yin P, Choi H M T, Calvert C R, et al. Programming biomolecular self-assembly pathways[J]. Nature, 2008, 451(7176): 318-322.<br><br> | |||
[3] Sharma J, Chhabra R, Andersen C S, et al. Toward reliable gold nanoparticle patterning on self-assembled DNA nanoscaffold[J]. Journal of the American Chemical Society, 2008, 130(25): 7820-7821.<br><br> | |||
[4] Ghosh P, Han G, De M, et al. Gold nanoparticles in delivery applications[J]. Advanced drug delivery reviews, 2008, 60(11): 1307-1315.<br><br> | |||
[5] Lermusiaux L, Sereda A, Portier B, et al. Reversible switching of the interparticle distance in DNA-templated gold nanoparticle dimers[J]. ACS nano, 2012, 6(12): 10992-10998.<br><br> | |||
[ | |||
[ | |||
[ | |||
< | |||
Latest revision as of 04:56, 27 October 2014
<html> <head> <meta http-equiv="Content-Type" content="text/html; charset=utf-8" /> <style type="text/css">
body {
display:block;
background-color:#f5f5f5;
}
.ys01 {
text-align: left;
}
/* --the header css -- */
- apDiv1 {
position:relative;
float:left;
left:0px; top:0px;
margin-right:0px;
width:1000px; height:360px; z-index:1;
box-shadow: 0px 5px 8px rgba(0,0,0,0.4);
overflow: visible; text-align: left; background-image: url(http://openwetware.org/images/3/37/Header_tianjin.png);
}
/* -- navigator css -- */
- apDiv2 {
position:relative; float:left;
left:0px;
width:1000px; height:40px; z-index:2;
box-shadow: 0px 5px 8px rgba(0,0,0,0.4);
font-family: Georgia, "Times New Roman", Times, serif; text-align: left; background-image: url(http://openwetware.org/images/thumb/7/76/Tianjin_index02.jpg/800px-Tianjin_index02.jpg);
}
- apDiv2 table {
font-size: 18px;
text-align: center;
}
.ys01 #apDiv2 table {
text-align: center;
}
.ys01 #apDiv2 table tr td a { color: #FFF;
}
/* -- navigator css -- */
- navDiv li { margin: 0; padding: 0;}
- navDiv ul {margin: 0; padding: 0; list-style: none; z-index:99;}
- navDiv a {text-decoration: none;margin: 0; padding: 0;}
- navDiv
{
/* -- background body of navigator css -- */
position: relative;
float: left;
height: 40px; width:1000px; left: 0px; background-color: #ffffff; background-repeat: repeat-x; box-shadow: 0px 5px 8px rgba(0,0,0,0.4); margin-bottom:40px;
z-index:60;
font-family:Georgia, "Times New Roman", Times, serif;
text-align: left;
}
- navDiv > ul > li {
float: left; margin-left: 30px; position: relative;
}
/* -- when mouse not on the navigator css -- */
- navDiv > ul > li > a {
color: #333333; font-family: Verdana, 'Lucida Grande'; font-size: 15px; line-height: 40px; padding: 11px 20px;
-webkit-transition: color .15s;
-moz-transition: color .15s;
-o-transition: color .15s;
transition: color .15s;
}
/* -- when mouse on the navigator css -- */
- navDiv > ul > li > a:hover {color: #ffffff; background-color: #000000;}
/* -- top and bottom of the sub meun css -- */
- navDiv > ul > li > ul
{
opacity: 0; visibility: hidden; padding: 16px 0 20px 0; background-color: rgb(250,250,250); text-align: left; position: absolute; top: 30px; left: 50%; margin-left: -90px; width: 180px;
-webkit-transition: all 0.3s 0.1s;
-moz-transition: all 0.3s 0.1s;
-o-transition: all 0.3s 0.1s;
transition: all 0.3s 0.1s;
-webkit-border-radius: 5px;
-moz-border-radius: 5px;
border-radius: 5px;
-webkit-box-shadow: 0px 1px 3px rgba(0,0,0,0.4);
-moz-box-shadow: 0px 1px 3px rgba(0,0,0,0.4);
box-shadow: 0px 1px 3px rgba(0,0,0,0.4);
}
- navDiv > ul > li:hover > ul
{
opacity: 1; top: 40px; visibility: visible;
}
- navDiv > ul > li > ul:before
{
content: ''; display: block; border-color: transparent transparent rgb(250,250,250) transparent; border-style: solid; border-width: 10px; position: absolute; top: -20px; left: 50%; margin-left: -10px;
}
- navDiv > ul ul > li { position: relative;}
/* -- when mouse not on the submeun css -- */
- navDiv ul ul a
{
color: rgb(50,50,50); font-family: Verdana, 'Lucida Grande'; font-size: 13px; background-color: rgb(250,250,250); padding: 5px 8px 7px 16px; display: block;
-webkit-transition: background-color .1s;
-moz-transition: background-color .1s;
-o-transition: background-color .1s;
transition: background-color .1s;
}
- navDiv ul ul ul
{
visibility: hidden; opacity: 0; position: absolute; top: -16px; left: 206px; padding: 16px 0 20px 0; background-color: rgb(250,250,250); text-align: left; width: 160px;
-webkit-transition: all .3s;
-moz-transition: all .3s;
-o-transition: all .3s;
transition: all .3s;
-webkit-border-radius: 5px;
-moz-border-radius: 5px;
border-radius: 5px;
-webkit-box-shadow: 0px 1px 3px rgba(0,0,0,.4);
-moz-box-shadow: 0px 1px 3px rgba(0,0,0,.4);
box-shadow: 0px 1px 3px rgba(0,0,0,.4);
}
- navDiv ul ul > li:hover > ul { opacity: 1; left: 196px; visibility: visible;}
/* -- when mouse on the navigator css -- */
- navDiv ul ul a:hover
{
background-color: #777777; color: rgb(240,240,240);
}
/* Back to top css */
.back-to {
position: fixed; bottom: 35px; *bottom: 50px; _bottom: 35px; right: -160px; z-index: 999; width: 50px; zoom: 1; }
- html .back-to {
/* 不能用 _position 这种写法,因为它在IE7+也会执行expression。。。 */
position: expression(function(ele){ele.runtimeStyle.position='absolute';Expressions.style.position.fixed(ele);}(this))
}
.back-to {
float: right; display: block; width: 50px; height: 50px; background: url(http://a.xnimg.cn/imgpro/button/back-home.png) no-repeat 0 0; outline: 0 none; text-indent: -9999em;
}
.back-to:hover {
background-position: -50px 0 }
.back-to .back-top {
float: right; display: block; width: 50px; height: 50px; background: url(http://a.xnimg.cn/imgpro/button/back-top.png) no-repeat 0 0; margin-left: 10px; outline: 0 none; text-indent: -9999em; }
.back-to .backtotop {
float: left; display: block; width: 50px; height: 50px; background: #666 url(http://a.xnimg.cn/imgpro/arrow/btt.png) 8px -57px no-repeat; margin-bottom: 15px; outline: 0 none; text-indent: -9999em; -moz-border-radius: 4px; -khtml-border-radius: 4px; -webkit-border-radius: 4px; border-radius: 4px; position: relative; -moz-box-shadow: 0px 0px 15px #ccc; -webkit-box-shadow: 0px 0px 15px #ccc; box-shadow: 0px 0px 15px #ccc; }
.back-to .backtotop:hover {
background-color: #333; background-position: 8px 13px; }
.back-to .backtotop .back-tip {
position: absolute; visibility: hidden; top: -31px; left: -10px; }
.back-to .backtotop:hover .back-tip {
visibility: visible; }
.back-to .back-top:hover {
background-position: -50px 0; }
- apDiv3 table {
text-align: center;
}
.ys01 #apDiv6 table { text-align: left;
}
.ys02 {
font-size: 36px;
color: #F00;
}
- apDiv10 {
position:relative;
float:left;
display:block;
left:0px;
width:960px;
box-shadow: 0px 5px 8px rgba(0,0,0,0.4);
z-index:3; text-align:left; font-family:Georgia, "Times New Roman", Times, serif; font-size: 18px; color: #000; background-color: #ffffff;
padding: 20px;
}
/*hidden section*/
.firstHeading{display:none;}
- sidebar-main{display:none;}
- p-cactions{display:none;}
- p-personal{display:none;}
</style> </head>
<body class="ys01";background-image: urlhttp://openwetware.org/images/0/0a/Paper.jpg);>
<!--header--> <div id="apDiv1"></div>
<!--navigator starts here-->
<div id="navDiv">
<ul>
<li class='active '><a href="http://openwetware.org/wiki/Biomod/2014/Tianjin"><span>Home</span></a></li>
<li class='has-sub'><a href="http://openwetware.org/wiki/Biomod/2014/wiki-2%27.html"><span>Idea</span></a>
<ul>
<li><a href='http://openwetware.org/wiki/Biomod/2014/wiki-2%27.html#background'><span>Background</span></a></li>
<li><a href='http://openwetware.org/wiki/Biomod/2014/wiki-2%27.html#motivation'><span>Motivation</span></a></li>
<li><a href='http://openwetware.org/wiki/Biomod/2014/wiki-2%27.html#calculation'><span>Calculation</span></a></li>
</ul>
</li>
<li class='has-sub'><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html"><span>Project</span></a>
<ul>
<li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#Three-arm locker"><span>Three-arm Locker</span></a></li>
<li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#Gold nanoparticle based photosensor"><span> Gold Nanoparticle Based Photosensor</span></a></li>
<li><a href="http://openwetware.org/wiki/Biomod/2014/experiment.html#dna origami"><span>DNA Origami</span></a></li>
</ul>
</li>
<li class='active'><a href="http://openwetware.org/wiki/Tianjin_protocol"><span>Protocol</span></a>
</li>
<li class='active'><a href="http://openwetware.org/wiki/Biomod/2014/future.html"><span>Future Work</span></a>
</li>
<li class='active '><a href="http://openwetware.org/wiki/Biomod/2014/members.html"><span>Members and Acknowledgement</span></a>
</li>
</ul> </div>
<div id="toolBackTo" class="back-to" style="left: 1175px; "> <a stats="site_footer_back_to_top" class="backtotop" href="#top" onclick="if(Sizzle('#sidebar2 .ready-to-fix')[0]) Sizzle('#sidebar2 .ready-to-fix')[0].style.position = 'static';window.scrollTo(0,0);if(Sizzle('#sidebar2 .ready-to-fix')[0]) Sizzle('#sidebar2 .ready-to-fix')[0].style.position = '';return false;">Back to top <img stats="site_footer_back_to_top" src="http://openwetware.org/images/a/a7/TJU2012-Back-tip.png" class="back-tip"> </a> </div>
<div id="apDiv10">
<a name="Three-arm Locker"></html>
Three-arm locker
<html></a></html>
DNA strand displacement
Strand displacement is the process through which two strands with partial or full complementarity hybridize to each other, displacing one or more pre-hybridized strands in the process[1]. The complementary single-stranded domains (referred to as toeholds) can be the initiator of the strand displacement which progresses through a branch migration process that resembles a random walk. By varying the strength (length and sequence composition) of toeholds, the rate of strand-displacement reactions can be quantitatively controlled over a factor of 106. More importantly, this feature allows engineering control over the kinetics of synthetic DNA devices.


Design
To approach the goals of local release and selective release, we should design the DNA locker which is expected to intelligently control the open and close of our device.
According to Peng’s paper[2], we designed a self-locking three-arm locker, which utilize strand replacement to assemble its secondary structures. However, we also optimize the three-arm structure with the irreversible characteristics of our lock, which means that it will be not re-locked once it breaks by heating.
This locker is composed of strand A, strand B, and strand C, acting as the main body of our lock and strand I, which plays the role of initiator.

First, the locking process is isothermal. It is capable to lock at constant room temperature with a simple displacement reaction when initiator I is added. The first step will occur with the exposed toehold of hairpin A nucleating with of I and opening the hairpin. The newly exposed single strand of A will nucleating with the exposed toehold of B and open the B hairpin. After that, the same reaction will happened and lead to the open of C hairpin binding to B. Then the final step occurs in which a single-stranded domain (a* of B) initiates a branch migration that displaces the initiator I from A.
When it comes to the characteristics of this structure, to get reach to our first goal, the tree-arm structure is supposed to be with high yield to form with the help of single-strand I and minimal leakage of a system containing only A, B and C.
And second, the locker should be irreversible. If we move single-strand I away and heat the locker, the double strands will melt, the locker opens, and A, B, C will return to independent hairpins. However, without I, even if the all the hairpins are annealed together, they cannot form the three-arm junction kinetically.
Third, we design the sequences of the three-arm structure with diverse melting temperature to reach the purpose of selective release which means to open different locks and release different drugs under various circumstances, so that we can make the decision of how much dose of drugs we release and which drugs.
Characterization and results

To realize those purposes of our design, we study Peng's paper and do some changes. Then modify and select the better groups step by step to get the optimum.
First, we try to repeat the experiment of Peng’s paper (Fig 1-3), and with the consequence we discover that with its high stabilization, the length of the sequence is a little bit long and the melting temperature is higher than our expect.
Modification
We modified the three-arm locker in the following 2 aspects.
First, in order to increase the yield of three-arm junction in the locking process and to increase the amount of independent hairpins in the unlocking process, we decided to change the number of base pairs in each domain to determine the proper lengths of the three arms. We synthesized 29 groups of various length lockers with numbers of base pairs in each domain varied from 3, 4, 5 and 6. Gel electrophoresis showed that the sequence with 4 bps per domain had the highest yield of three-arm junction in the locking process and relatively low leakage. So we reached the conclusion that 4 bps per domain is the proper length for each arm of the locker to suit our design. And we pick up the comparatively best group M4-11 to do further research.
Second, we wanted to get a group of lockers which can open in response to different melting temperature to reach the selective release, so we choose to change the length of the complementary sequence to get different melting temperature. And based on the group M4-11, we would further modify one of the domains specifically. Domain a,b and c are beyond our choice because act as toehold, the modification of them will greatly affect the formation rate of our locker. Domain x,y are also excluded because they are in strand A, and modification of them calls for using different initiator I which makes the selective released system more complicated and difficult to form. We finally chose to modify domain z which has the least effect on the whole structure.
We synthesized 11 groups of lockers with group M4-11 and changed the number of base pairs in domain z within the range of 4,6,8,10. Finally, we selected out a high-yield gradient of lockers with different z domain of 4,8,10 bps named M4-11(Group1), M4-11-8-04(Group2), M4-11-10-01(Group3). We also heated the lockers in a temperature range from 50℃ to 65℃ and they could open successfully. As the results we show below(Fig 1-4)(Fig 1-5)
And to get comparatively accurate melting temperature of Group1,2,3, we also heating in smaller range of temperature(Fig 1-6).


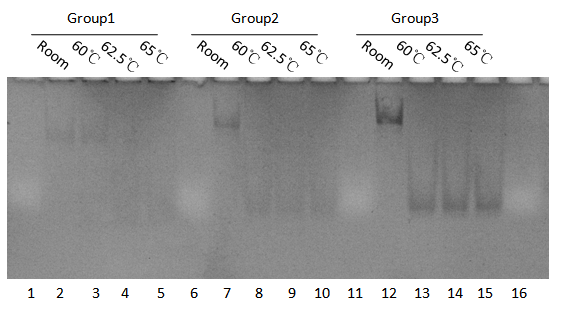
Gel electrophoresis confirms:
1.The yield of the locker is high with I added.
2.Without I, at constant temperature, the yield of the locker is relatively low,which means leakage is quiet low for these three groups.
3.Without I, annealed the hairpins A, B and C, the yield of locker is also low. So when the double strands are heat and separated, it could hardly to pair again without the initiator I.
<html><a name="Gold nanoparticle based photosensor"></html>
Gold Nanoparticle Based Photosensor
<html></a></html>

Functionalization of thiolated DNA on gold nanospheres was carried out by following a previous published protocol with some modifications. Briefly, the disulfide bond in the thiol-modified oligonucleotides was reduced to monothiol using TCEP (10mM, 1h) in water to activate the thiolated DNA. The DNA-SH sample was added drop by drop into the gold nanoparticle solution. The solution was then incubated for 16 h at room temperature. Finally, the resulting solution was centrifuged at 1500g for 60 min and then the supernatant was decanted to remove unbound DNA-SH.
We use gel electrophoresis to test the DNA-GNPs. The electrophoresis experiments were performed in 2 % agarose gels at 100 V,1 h. We used 5X TBE as running buffer. Gold colloids in this size range have a deep red color and can be easily visualized in the gels. The mobility of particles on the gel depends on two factors: size and charge. The bigger the size, the slower and the higher the charge, the faster particles will migrate. In the case of negatively charged Au particles, the attachment of negatively charged DNA molecules causes in first place an increase of size that can be seen as a retardation of the band of the gel. Upon attachment of DNA, the mobility of the resulting conjugates was always moderately decreased. Therefore, in agreement with previous reports, we assume throughout this manuscript that attachment of DNA to Au nanoparticles in first order increases the effective diameter of the conjugates which can be directly seen in the retardation of the band of the conjugates in gel electrophoresis experiments. The bands of DNA-Au NP complex will move slower than those of pure Au nanoparticles.
The bands can be extracted from the gel by cutting out the agarose piece that contains the band and immersing it into 5×TBE buffer solution. After two days, the Au-DNA conjugates could be diffused out of the gel into the buffer. The extraction procedure ensures that all DNA is really attached to the Au particles, since free DNA migrates in a much faster band.
For the purpose of assembling the origami-GNPs complex,i.e. attaching GNPs to DNA origami as illustrated in Figure 1, we use thiol-modified oligonucleotides (short synthetic DNA sequences), which can be loaded onto the surface of GNPs to combine GNPs and DNA. GNPs are fixed onto the DNA origami by linking them to staple strands whose 5’end are modified with lipoic acid. Upon hybridization between DNA tails on DNA origami and single staple strands on GNPs, GNPs are attached to the DNA origami, resulting in the formation of origami-GNPs complex. DNA origami is used for in vivo delivery of chemotherapeutic drugs in our project. Originally, the photothermal property of GNPs was used in biologically relevant studies to destroy cancer cells, while, in our project, it has been harnessed as a means to optically elicit the release of drugs encapsulated in DNA origami.

In order to know gold nanoparticles of which concentration can be easily visualized in the gels, we diluted 13nm gold nanoparticle solution with water of different volumes (the volume ratios of gold nanoparticle solution and solution are 1/1,1/10,1/25,1/50,1/100,respectively). And the result showed that when the the volume ratio is at least 1/25, the diluted 13nm gold nanoparticle solution will show a clear red band in gels.


Then we compared the bands of 5nm and 10nm gold nanoparticles. From the figure we could see that the bands of 5nm gold nanoparticles are single while those of 13nm gold nanoparticlse are continous. This is probably because gold nanoparticles are erratic in TBE buffer while we used 5X TBE as the gel running buffer. And our 5 nm gold nanoparticles were purchased from sigma-aldrich and 13 nm gold nanoparticles were synthesized by citrate reduction of HAuCl4. Hence the stability of our 5nm gold nanoparticles may be better than 13nm gold nanoparticles.
<html><a name="dna origami"></html>
DNA Origami
<html></a></html>
Design
We want to design a simple two-dimensional DNA origami rectangle which can be folded by our three-arm DNA locker into a roll. The dimensions of the origami plate are properly selected so the drug stays encapsulated inside it. The final dimensions are the following:
Length: 50 nm
Width: 35 nm
Thickness: 2 nm

To enable the origami to fold into a roll, the three arm locker should be combined with it. The staple strands in two corners of the origami plate are extended with the sequences of strand A, and the staple strands in the other two corners are extended with the sequences of strand C. Strand A and strand C comes from our three-arm DNA locker M4-11-6-01(Group 3) and the one with best isothermal and irreversible properties. We used the cadnano 2 program for our rectangle shape origami design.
The scaffold used in this design is M13mp18 single strand DNA with 7249 nt, from New England Biolabs. The desired structure and dimensions are achieved with the help of two types of staples:
• core: 58 staples give stability to the whole structure.
• edge: 4 staples give stability to the edges and with extending sequences of A or C.

Folding and characterization
The mixture of scaffold and different staples were subjected to a thermal annealing process composed of 3 steps that allowed the folding. Different initial temperatures varying from 50.0℃ to 65.0℃ in step 2 were tested. Electrophoresis showed that low initial temperatures in step 2 have relatively high origami yield.(Fig.3-1)


References
[1] Zhang D Y, Seelig G. Dynamic DNA nanotechnology using strand-displacement reactions [J]. Nature chemistry, 2011, 3(2): 103-113.
[2] Yin P, Choi H M T, Calvert C R, et al. Programming biomolecular self-assembly pathways[J]. Nature, 2008, 451(7176): 318-322.
[3] Sharma J, Chhabra R, Andersen C S, et al. Toward reliable gold nanoparticle patterning on self-assembled DNA nanoscaffold[J]. Journal of the American Chemical Society, 2008, 130(25): 7820-7821.
[4] Ghosh P, Han G, De M, et al. Gold nanoparticles in delivery applications[J]. Advanced drug delivery reviews, 2008, 60(11): 1307-1315.
[5] Lermusiaux L, Sereda A, Portier B, et al. Reversible switching of the interparticle distance in DNA-templated gold nanoparticle dimers[J]. ACS nano, 2012, 6(12): 10992-10998.
<html> </div> </html>