Bioreactors for Tissue Engineering, by Varun Chalupadi, Anthony Sanford and Blayne Sarazin
Definition
Bioreactors are defined as a device used to develop biological processes by closely monitoring controlled environments [1]. Bioreactors are able to be tuned precisely to meet the needs of the cells to be cultured within them. Many cells require a certain temparature, pH, and even mechanical forces to facilitate optimal differentiation and proliferation . Bioreactors allow for control of all of these factors and more. They are used within the field of tissue engineering as a method for studying and mimicking traditional in vitro studies in an in vivo environment for the growth of tissue substitutes [2]. Typically, bioreactors are used in the scale up process in transferring from bench-scale lab experiments to large-scale production schemes.
Bioreactors are developing solutions to common problems associated with non-bioreactor implemented solutions. Most notably, effective transfer of nutrient supply to the cell culture is one of the major concerns addressed using a bioreactor. Additionally, the bioreactor provides a well-defined culture condition than in vivo tissue generation as multiple parameters can be controlled [2].
Today, bioreactors are used widely to produce various tissue cultures including vocal fold, retinal, skin, muscle, ligament, tendon, bone, cartilage and liver [2].
History of Bioreactors
1918 - Biochemist and former Israeli President Chaim Weizmann developed a bacteria fermentor for the production of acetone.[3]
1944 - De Beeze and Liebmann used the first large scale (above 20 litre capacity) fermentor for the production of yeast.[3]
1949 – New Brunswick Shaker is born.[4]
1952 – Streptomycin isolated creating an instant high demand for NBS
1960s – Reciprocating water bath and refrigerated shakers provide rigorous mixing, and temperature conrol
1970s – First commercial fermenters, autoclaves, freeze dryers, colony counters are developed
1980s – First benchtop bioreactor, the CelliGen is developed, designed for animal cell culture [4]
1990s – Microprocessor controlled shakers, provide precision control of setpoints, alarms, running time, agitation, pCO2, and temperature [4]
1990 - First rotary cell culture system invented by NASA and introduced commercially by Synthecon.[5]
2001 – Benchtop system can control up to 4 bioreactors, controller enables users to view and change process parameters [4]
2005 – Labor-saving system uses just one to four inexpensive, disposable cell culture flasks to produce yields equivalent to dozens of spinners, or hundreds of T-Flasks or rollers
2016 – Reverse Membrane bioreactor design is introduced to tackle issues in bioconversion and fermentation feeds that contain high concentrations of inhibitory compounds.
2016 - Bioreactor design introduced to enhance the production of tissues with high cell densities and enhanced mechanical properties, aiming to construct a bioreactor able to host cell colonization and growth within 3-D printed nose scaffolds[6].
Controlled Parameters for Bioreactors
There are some parameters that must be controlled and appropriately adjusted in order to maintain controlled, reproducible experiments. The parameters are:
- Temperature
- pH
- Oxygen Diffusion
- Nutrient transport
- Waste Removal
- Temperature
These parameters are crucial to maintain in a properly engineered bioreactor. Specifically, mass transport and diffusion limitation problems related to oxygen transport have “severely curtailed efforts to engineer tissues that normally have high vascularity and/or cellularity”[2].
Types of Bioreactors
Spinner Flasks

A Spinner flask is a basic form of a tissue-engineered bioreactor that is ideal for cultures cultivated under static conditions. An advantage of the spinner flask design is it maintains a well-mixed environment within the flask, which therefore reduces the stagnant layer of cells what would form in a poorly mixed environment. Conversely, spinner flasks are not always ideal since the constant mixing motion causes turbulent flow within the capsule and the associated high shear stress causes the formation of an outer fibrous capsule in the cartilaginous tissue.
Spinner flasks have been modified (2004) since the original design to reduce the turbulent flow. Current designs called wavy-walled bioreactors induce small waves in the mixing instead of the rough, turbulent flow induced from traditional spinner flasks.
Spinner flasks are intended for small scale production and do not appear to be used as much as other types. They are primarily used for the seeding of cells in 3D scaffolds until they are ready for more large-scale cell culture procedures [1].

Perfusion Systems
The perfusion system offers an alternative to the spinner flask technology as it combats the problems related to the static culture conditions. Typically, static media does not provide optimal cell growth and thus deters the cell’s ability to migrate into the scaffold. This results in a shell formation of cells on the exterior of the scaffold instead of a desired uniform distribution [2]. Perfusion systems constantly replenish the cells with fresh media with the use of chambers, columns, or cartridges that house the scaffold constructs [2]. The constant replenishment of media enhance nutrient delivery to the cells and help the cells to better differentiate. One of the proposed disadvantages of the perfusion system is the constant flow of media causes the cells to line up parallel to the scaffold lining while in certain systems, it would be preferred to orient the cells perpendicularly, such as articular cartilage [2].
A company called Tissue Genesis has created a special kind of perfusion system bioreactor called the Bio-Optimized System (BOS). This system employs the use of sensors, actuators, pumps, disposable chambers, and a perfusion system to cultivate vascular grafts and connective tissues with high efficacy due to the use of feedback-controlled hardware [2]. Perfusion systems are also used in the pharmaceutical industry to generate genetic therapies that use cell cultures for protein and enzyme replacement therapies.
Rotating Wall Vessel (RWV)
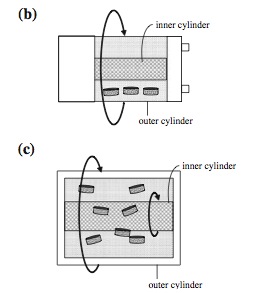
One of the challenges in designing an ideal bioreactor is reduction of shear stress within the capsule. The rotating wall vessel (RWV) is the most commonly used bioreactor that reduces shear stress since the cells are grown in a microgravity environment [2]. The RWV was originally invented by NASA and currently has several derivatives of the original design that are implemented today. There are three common derivatives called the slow lateral turning vessel (STLV), the high aspect ratio vessel (HARV) and rotating wall perfused vessel (RWPV) [2]. The STLV is currently available for commercial production.
The rotating wall structures provide a reduced shear stress while maximizing mass transfer due to the microgravity environment generated by the rotation of the inner and outer cylinders [1]. The rotation establishes a equilibrium of all three dynamic forces involved [2]. The major disadvantage to the RWV design is that the cell growth is not always uniform. Due to the rotating action of the cylinders, the scaffolds within the capsule tend to bounce off of the reactor wall causing cell damage along the outside edges of the scaffolds [2]. Newer techniques have found ways of alleviating this problem by using lighter scaffolds which do not bounce off the reactor walls as hard [2].

Algae Bioreactors
Algae bioreactors, also referred to as photobioreactors, are used for the intentional cultivation of algae to fix carbon dioxide and/or produce biomass (organic matter used as biofuel). One of the main tools that helps algae grow within a bioreactor is aeration. A common bioreactor for this specific application is called a bubble column bioreactor.[8] Aeration is a key aspect to growing algae within a bioreactor because it helps replicate real-life conditions (as do many bioreactors). A bioreactor at the National Renewable Energy Laboratory (NREL) called the Simulated Algal Growth Environment (SAGE) can precisely control light, temperature, and delivery of carbon dioxide such that it can mimic many different types of environmental conditions. For example, if the researchers wished to know how algae would grow in different types of water, the SAGE can be set up to simulate these water conditions, ultimately allowing researchers to asses algae growth in a variety of environmental settings. The research in this lab employ a "feed/starve" approach to maximize the yield from the algae. The key to this approach is throttling the amount of nitrogen available, cause the algae to enter into a sort of storage mode, rather than continuing to multiply. The algae turns to storing the carbon dioxide in finds in its environment, and begins to build mass. This mass is made entirely out of carbohydrates and oils, which is the key to using algae bioreactors to obtain biofuels. The goal of this bioreactor is grow algae in such a way that the algae produces large amounts of proteins and carbohydrates that can then be turned into diesel, butanol, and other biofuels. This work was published in 2014. [7]
For more information on NREL, follow this link: https://www.nrel.gov/bioenergy/
Other bioreactors from other organizations, such as NASA, have been introduced to do similar designs as the SAGE reactor. Algae bioreactors are mainly utilized when the harvesting of biofuels is the concern of the researchers.
Yeast Bioreactors (Fermentors)
Like algae, yeast bioreactors are also implemented for the reproduction and cultivation of biomass. While the biomass that is produced from algae is often in the form of proteins and carbohydrates that can be used as biofuels, the biomass produced from yeast is instead used as a human or animal protein supplement in our food. A study published in 2013 implemented a glucose-limited feeding strategy for yeast biomass production in a bubble column reactor. Bubble column reactors are also used in the very same way for algae. For this study, the researchers developed a model that was able to increase previously reported biomass yields of yeast 6.4 fold. However, the study struggled due to the small size of the bubble bioreactor, theorizing that an even larger, industrial-sized bioreactor would facilitate higher degrees of biomass production.[9]

Bacteria Bioreactors
As mentioned in the history section, one of the first ever applications of a bioreactor was by Chaim Weizmann, in which he utilized bacteria as a bioreactor create acetone, butanol, and ethanol. The process he developed became known as the acetone-butanol-ethanol (ABE) fermentation process. This was a process that used bacteria fermentation of a way to produce acetone for the British during World War 1.[3] The process is similar to how yeast ferments sugar to produce ethanol for alcohols and fuel. The ABE fermentation process produces its solvents in a 3:6:1 ratio, respectively. It typically utilizes a strain of bacteria for the Class Clostridia. Clostirdium acetobutylicum is the most well-studied and widely used species, however, Clostridium beijerinckii has also been utilized and has been shown to produce decent results.[10] While there are currently are no operating ABE plants, ABE fermentation is getting a second look for the possibility of using butanol as a biofuel. More recent work involving an E. Coli. bioreactor has been introduced. Research from the Robert Woof Johnson Medical School in New Jersey utilized a bacterial system that implements a combination of low temperature and induction of mRNA-specific endoribonuclease to influence high level protein expression.[11]
Companies That Sell Bioreactors
Bose
Bose is relatively new to the realm of biotechnology, with the development of their perfusion

bioreactor in 2014. This bioreactor is optimized for general tissue regrowth situations, as it can be used for different sample sizes and types, including bone, blood vessels, skin, ligaments, tendons.[12] The bioreactor itself is equipped with a multi-chamber configuration that can hold up to six chambers, which can be oriented individually, either vertically or horizontally. It can be set up as a single chamber with a specimen fixture or as a complete, multi-chamber apparatus. This bioreactor is available in two price increments, with the single chamber setup costing $4500 while the multi-chambered setup will generally cost ~$12,500. Note: this is just the cost for the chamber setup themselves. The price for the entire apparatus is likely thousands of dollars more. Most companies do not offer the price outright, so a quote is required to get an actual dollar amount for the whole system. Other costs can include the base, stimulators, pumps, software, controllers, consumables, and other miscellaneous items.
The bioreactor can be viewed on the Bose site by following this link: http://pdf.directindustry.com/pdf/bose-electroforce-systems-group/bose-3dculture-pro-bioreactor/50709-583914.html
Biss
Biss is a bioreactor powerhouse that has a multitude of bioreactor setups. They offer up a variety of bioreactors with a focus of imparting mechanical stresses onto scaffolds. While other bioreactors are merely for enhanced nutrient transport, Biss bioreactors often include perfusion/compression systems that facilitate compression and shear stress without sacrificing nutrient transport. The loading conditions of these bioreactors include: compression, tension, pressure, and shear. Biss also cells bioreactor setups that have very tissue-specific applications. Here are some of those bioreactors:
- LumeGen & CardioGen: Pulsatile Pressure and Flow Bioreactor - Specializes in pulsatile flow and shear stress to a vascular construct.
- LigaGen: Tension Bioreactor - Provides axial stress stimulation to tendons and ligaments.
- CartiGen: Compression Bioreactor - Applies oscillatory axial stress and perfusion to cartilage constructs.
- Other tissue-specific bioreactors Biss has to offer include the OsteoGen and DermiGen which are linked to bone and skin applications, respectively.
Like other bioreactor websites, prices are not included unless you obtain a quote. However, the Lynch Lab at the University of Massachusetts - Amherst uses an OsteoGen bioreactor which reportedly cost ~$100,000 for all items included within the setup.
To view some of these bioreactors, follow this link to the Biss Bioreactor website: http://www.tissuegrowth.com/prod_systems.cfm
ThermoFisher Scientific
ThermoFisher scientific is well known as one of the biggest suppliers of lab equipment, so it comes as no surprise that they have their own group of bioreactors. Referring to them as Single-use Bioreactors, ThermoFisher offers many different sizes of stirred-tank bioreactors in order to support large-scale cell growth. These stirred-tanks come in a variety of sizes ranging from 50 L - 2,000 L tanks that are optimized for batch, fed-batch, and perfusion cell cultures. ThermoFisher also offers what they call a Single-Use Fermentor for microbial fermentation with emphasis on the ability to control mass transfer, mixing, and temperature. All tanks are stirred with motor-powered impellers that facilitates constant mixing, effectively increasing the amount of nutrient and mass transport where necessary. No prices are immediately available when visiting the site, a quote is necessary to acquire a price.[13]
To learn more about bioreactors offered by ThermoFisher Scientific, follow this link: https://www.thermofisher.com/us/en/home/life-science/bioproduction/single-use-bioprocessing/single-use-equipment/single-use-bioreactors.html

Why Bioreactors are Necessary for Tissue Engineering

Homogeneous cell distribution can be achieved in the scaffold by using a bioreactor. Enhancing fluid transport is essential for proper viability throughout the scaffold. Cells also require mechanical stimulation and bioreactors can be used to apply the necessary stimulation to promote ideal growth. This can encourage increase the rate of ECM production and result in a more homogeneous distribution than would be the case with static culture. For example, in comparisons between ECM protein levels after 5 weeks in culture, scaffolds cultured under hydrodynamic conditions showed significant improvements over scaffolds cultured in static medium [14].

A study by Martin et al was performed to test the hydrodynamic conditions obtained from different bioreactor conditions for cartilage tissues after sitting in culture for six weeks. They were able to test two different conditions to test for the production of glycosaminoglycan (GAG) [1].
The initial control was a static culture construct. In the first illustration, it is evident that GAG was able to be produced in the outer regions but do appear in the central regions of the tissue [1]. The first test was to use the spinner flasks for the tissue culture. In this culture it is evident that the media mixing allows for better production of GAG through the central areas of the tissue. It is clear that there is a more level distribution of the GAG production in the cartilage tissue cells. The second test was the implementation of a RWV bioreactor. In this test, it is evident that the lack of shear stress and better mixing at equilibrium generated a much more intense GAG concentration throughout the tissue sample [1].
It is evident form this test that the use of bioreactors provides a better cellular environment for the cells to survive and produce the appropriate GAG protein. The comparison of the spinner flask and RWV system clearly illustrates that a perfused RWV system is much better than due to the low shear stress and better media uptake.
Tissue Engineered Bioreactor Products


Dermagraft
Artificial skin is one of the well-known tissue engineered products created with the use of bioreactors. TransCyte and Dermagraft are two artificial skin products that employ the use of a modified perfusion system to culture the cells. TransCyte and Dermagraft are dermal tissue replacement solutions developed for use in diabetic foot ulcers and [16]. Dermagraft is produced using roller bottle technology in which the cells adhere to the bottle surface during the initial expansion phase [17]. During this phase, up to 100 roller bottles can be used over five weeks for the expansion. Then manifolds with the graft are constructed and the cells and fresh media are constantly replenished over the scaffold until it is seeded with a high concentration of cells [17].
The following link from the Dermagraft website has a nice video of the manufacturing and scientific background of the product: http://www.dermagraft.com/about/product-videos/
AlloSource
AlloSource is a company currently producing allografts or bone graft for damaged bone tissue. A study performed by Zhang et al illustrated that the RWV bioreactor was the only type of bioreactor that was successfully able to produce bone grafts from mesenchymal stem cells [18]. AlloSource holds one of the largest tissue banks in the United States and they are responsible for creating safe bone and soft tissue replacements throughout the body. Their products range in allografts for all sections of the body. Some of the bone grafts they produce are shown in the pictures [15].
Current Bioreactor Specifications
CellekBiotek U-CUP [19]
<html> <iframe width="560" height="315" src="//www.youtube.com/embed/kcwn5FOOBgM" frameborder="0" allowfullscreen></iframe> </html>
- Rack hosts up to 10 independent U-CUP bioreactors and fits into an incubator
- Rigid or soft scaffold; ceramic, synthetic or natural polymer based
- Scaffold thickness:2–4 mm; Diameter:6-10 mm
- Working Volume: 6-14 mL
- Viable Cell Density: ~10x106 cells/mL
TissueGrowth DynaGen [20]
<html> <iframe width="560" height="315" src="//www.youtube.com/embed/PUBetmkKv4Y" frameborder="0" allowfullscreen></iframe> <iframe width="560" height="315" src="//www.youtube.com/embed/ELGaWD2i9nw" frameborder="0" allowfullscreen></iframe> </html>
- Chamber can accommodate a single scaffold
- Length of 45-70 mm,
- Width of 20 mm
- Thickness of 2 mm
- Working Volume of 60 mL - 100 mL
Current Research in Bioreactors
Two studies that came at the end of 2016 involve the design and implementation of new bioreactor setups.

Reverse Membrane Bioreactor - October 2016
The first study involves the design of a reverse membrane bioreactor built to assist with cell retention to maximize biofuel production.[21] The motivation for this work comes from the desire to reduce greenhouse gas emissions by producing fuel alternatives to fossil fuels. The researches argue that traditional membrane bioreactors (MBRs) are inefficient when handling feed sources that contain high amounts of cell inhibitory compounds or different prioritized substrate sources. Furthermore, traditional MBRs struggle with high suspended solid content that causes a cake layer formation and overall membrane fouling. The researchers propose their design of reverse membrane bioreactor (rMBR) to account for these shortcomings. The main difference between the two is that cells are immobilized in between membrane layers separated from feed medium, rather than outside a membrane layer in direct contact with medium. The results showed that rMBRs exhibited superior properties such as high local cell density, increased diffusive nature of compound separation and the ability of cell separation and reuse. The researchers conclude that this new technology has the potential for the bioconversion of more complex substrates, allowing for increased yields of biofuels in future studies. [21]

[[Image:nose_reactor.PNg|thumb|left|250px|
Bioreactor for Engineering Articular Cartilage based on 3-D Printed Scaffolds - December 2016
This study, which came from the Aristotle University of Thessaloniki in Greece, focuses on the regrowth of articular cartilage on a 3-D printed nasal septum-like scaffold to subsequently be used as an implant for patients who have experienced cartilage injury or degeneration specifically related to the nose.[6] The scaffold used for these experiments was a nose septm cartilage-like scaffold that was 3-D printed from Poly-lactic acid (PLA). This scaffold was seeded with human mesenchymal stem cells (hMSCs) and with the idea being that the bioreactor influences differentiation into chondrocytes. However, conventional bioreactors lacked the specifications necessary to conduct tests on such a scaffold, so the researchers designed their own bioreactor that is a combination of a spinner flask and a rotating wall bioreactor. While the paper does not say whether or not the bioreactor was able to effectively grow cartilage on the septum scaffold, the paper does say that future design iterations of the bioreactor will include that ability to compress the scaffold to impart greater mechanical stresses on it.
The Future of the Bioreactor
As bioreactor technology develops into a much more prolific technology, researchers aim to improve the power behind single bioreactor systems. The following diagram is a rendition of what the bioreactor of the future would look like.

The central idea is to make an all-encompassing “bioreactor” that can be available onsite for the surgeons to have access to. The idea behind this “bioreactor” is to allow the surgeon to perform a biopsy and inject the sample into the reactor. The media and nutrient supplies should be held on board or fed directly into the bioreactor. This all-encompassing system will have the ability to automatically isolate and expand the cells and seed them on a scaffold. Additionally, the system will be able to produce a graft and perform all required tests on the newly engineered device to output a device ready for implantation [1].
This closed system design is the vision for the next generation bioreactors and would eliminate the need for large GMP facilities by minimizing handling time and therefore minimizing contamination. Currently the costs associated with this design is simply too expensive to implement.
References
-
Martin I., Wendt D., Heberer M. The role of bioreactors in tissue engineering (2004) Trends in Biotechnology, 22 (2), pp. 80-86.
-
Chen C., Hu Y. Bioreactors for tissue engineering (2006) Biotechnology Letter, 28, pp. 1415-1423.
-
(Bioreactor): History, Design and Its Construction." Biology Discussion. N.p., 16 Sept. 2016. Web. 19 Apr. 2017.
-
BIOREACTORS IN BIOTECHNOLOGY - History". Bioreactors.weebly.com. N.p.
-
Rayl, A. J. S. "The Spin on Rotary Culture." The Scientist. N.p., 28 Oct. 2002. Web. 20 Apr. 2017.
-
Theodoridis, Konstantinos. Design of a laboratory bioreactor for engineering articular
cartilage based on 3D printed nasal septum-like scaffolds. Aristotle University Medical Journal. Vol 43, No 2 (2016)
-
Scanlon, Bill. "Unique Bioreactor Finds Algae's Sweet Spot." Unique Bioreactor Finds Algae's Sweet Spot - News Feature | NREL. National Renewable Energy Laboratory, 18 Feb. 2014. Web. 22 Apr. 2017.
-
Jakobson, Hugo A. Bubble Column Reactors. Chemical Reactor Modeling pp 757-806
-
Vieira, Eirka D. Yeast Biomass Production: A New Approach in Glucose-Limited Feeding Strategy. Braz J Microbiol. 2013; 44(2): 551–558.
-
Qureshi N, Blaschek, HP. 2001. Recent advances in ABE fermentation: hyper-butanol producing Clostridium beijerinckii BA101. J Ind Microbiol Biotechnol 27(5):287-291.
-
Suzuki, Motoo. Bacterial Bioreactors for High Yield Production of Recombinant Protein. Journal of Biological Chemistry. 2006; 281: 37559-37565.
-
Bose® 3DCulturePro™ Bioreactor Provides Reliable Tissue Growth from a Versatile, Easy-To-Use System." Bose Global Press Room - New Bose® 3DCulturePro™ Bioreactor Provides Reliable Tissue Growth from a Versatile, Easy-To-Use System. Bose, 12 Nov. 2014. Web. 19 Apr. 2017.
-
"Single-Use Bioreactors." Thermo Fisher Scientific. N.p., n.d. Web. 22 Apr. 2017.
-
Plunkett, N., & O'Brien, F. J. (2011). Bioreactors in tissue engineering. Technology and Health Care, 19(1), 55-69.
-
AlloSource. "Allograft Catalog." Allografts. AlloSource, 2012. Web. 08 Mar. 2012. <http://www.allosource.org/medical-professionals/allografts>.
-
Advanced Biohealing Inc. "TransCyte | Human Fibroblast-derived Temporary Skin Substitute | TransCyte.com." TransCyte. Advanced Biohealing, 2010. Web. 08 Mar. 2012. <http://www.transcyte.com/>.
-
Zhi-Yong Zhang, Swee Hin Teoh, Erin Yiling Teo, Mark Seow Khoon Chong, Chong Woon Shin, Foo Toon Tien, Mahesh A. Choolani, Jerry K.Y. Chan, A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering, Biomaterials, Volume 31, Issue 33, November 2010, Pages 8684-8695, ISSN 0142-9612, 10.1016/j.biomaterials.2010.07.097. <http://www.sciencedirect.com/science/article/pii/S0142961210009695>
-
Cellek Biotek AG. "U-CUP |Details |Video." Cellek Biotek. U-CUP. 2012. Web. 8 Apr 2014. <http://www.cellecbiotek.com/pages.aspx?lang=en&id=83>.
-
Tissue Growth Technology. "DermiGen Bioreactor." TGT. DynaGen Series. Dermigen. 2013. Web. 8 Apr 2014. <http://tissuegrowth.com/prod_skin.cfm>.
-
Mahboubi, Amir. Reverse Membrane Bioreactor: Introduction to a New Technology for Biofuel Production. Biotechnology Advances Volume 34, Issue 5, September–October 2016, Pages 954–975
-
Advanced Biohealing Inc. "Product Videos." Dermagraft. Advanced Biohealing, 2010. Web. 08 Mar. 2012. <http://www.dermagraft.com/about/product-videos/>.
-
Lei, Y., & Schaffer, D. V. (2013). A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proceedings of the National Academy of Sciences, 110(52), E5039-E5048.
-
Medscape. "Bone Graft Substitute Materials." Medscape. WebMD L.L.C., 16 Feb. 2012. Web. 7 Mar. 2012. <http://emedicine.medscape.com/article/1230616-overview>.
