Birbach:Research: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
==Inflammation in prostate tumorigenesis== | ==Inflammation in prostate tumorigenesis== | ||
This project is funded by the Austrian Science fund [http://www.fwf.ac.at FWF], project [http://www.fwf.ac.at/en/abstracts/abstract.asp?L=E&PROJ=P21919 P21919-B13]. | This project is funded by the Austrian Science fund [http://www.fwf.ac.at FWF], project [http://www.fwf.ac.at/en/abstracts/abstract.asp?L=E&PROJ=P21919 P21919-B13]. | ||
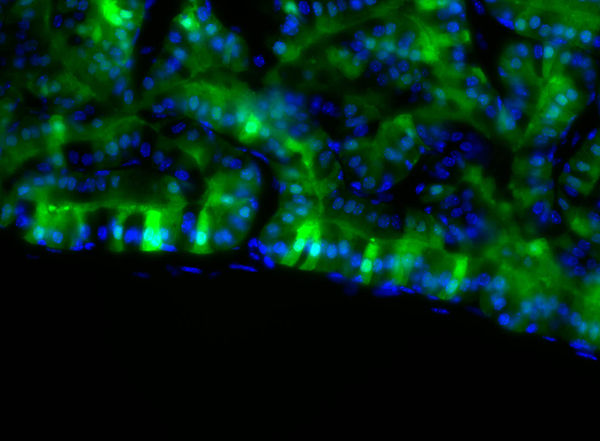
Inflammation is thouht to have a causative role in about 20% of human cancers. In prostate cancer, epidemioloical data sugest a link between chronic inflammation and early events in tumorigenesis, although a proof of this concept in a genetic model is lacking. In this project, we have established a mouse model combining monoallelic loss of the tumor suppressor PTEN together with expression of a constitutively active version of the IkappaB kinase 2 (IKK2) in prostate epithelial cells. We find that these double transgenic mice exhibit proliferative neoplasia (PIN) with large tumors, in contrast to non- | |||
Inflammation is thouht to have a causative role in about 20% of human cancers. In prostate cancer, epidemioloical data sugest a link between chronic inflammation and early events in tumorigenesis, although a proof of this concept in a genetic model is lacking. In this project, we have established a mouse model combining monoallelic loss of the tumor suppressor PTEN together with expression of a constitutively active version of the IkappaB kinase 2 (IKK2), a key mediator in the central inflammatory NF-kappaB pathway, in prostate epithelial cells. We find that these double transgenic mice exhibit proliferative neoplasia (PIN) with large tumors, in contrast to non-enlarged single transgenic (monoallelic PTEN or IKK2ca-only) prostates. This phenotype correlates with persistent inflammation of the prostate tissue, characterized by infiltrating granulocytes and macrophages. Molecularly, the inflammatory phenotype is due to expression of inflammatory chemokines and cytokines in the double transgenic epithelium. | |||
Strikingly, smooth muscle is lost around neoplastic prostate ducts. In human tumors, loss of smooth muscle is lost in carcinomas, and absent smooth muscle cell staining is regarded as an indication for invasive tumor formation in some prostate tumor models. However, in our model we show that loss of smooth muscle is just one step in the progression of the tumor, with a subsequent step necessary for carcinoma formation. | |||
In the epithelium, proliferative growth correlated with downreulation of tumor suppressor Nkx3-1. Thsi genes is positively regulated by the androgen receptor; in inlfamed prostates, androgen receptor activation was decreased, offering a potential explanation for hyperplastic changes. | |||
See our [http://www.neoplasia.com/pdf/manuscript/v13i08/neo11524.pdf publication] in Neoplasia for details. | |||
Revision as of 02:33, 16 August 2011
}
Projects
Inflammation in prostate tumorigenesis
This project is funded by the Austrian Science fund FWF, project P21919-B13.
Inflammation is thouht to have a causative role in about 20% of human cancers. In prostate cancer, epidemioloical data sugest a link between chronic inflammation and early events in tumorigenesis, although a proof of this concept in a genetic model is lacking. In this project, we have established a mouse model combining monoallelic loss of the tumor suppressor PTEN together with expression of a constitutively active version of the IkappaB kinase 2 (IKK2), a key mediator in the central inflammatory NF-kappaB pathway, in prostate epithelial cells. We find that these double transgenic mice exhibit proliferative neoplasia (PIN) with large tumors, in contrast to non-enlarged single transgenic (monoallelic PTEN or IKK2ca-only) prostates. This phenotype correlates with persistent inflammation of the prostate tissue, characterized by infiltrating granulocytes and macrophages. Molecularly, the inflammatory phenotype is due to expression of inflammatory chemokines and cytokines in the double transgenic epithelium.
Strikingly, smooth muscle is lost around neoplastic prostate ducts. In human tumors, loss of smooth muscle is lost in carcinomas, and absent smooth muscle cell staining is regarded as an indication for invasive tumor formation in some prostate tumor models. However, in our model we show that loss of smooth muscle is just one step in the progression of the tumor, with a subsequent step necessary for carcinoma formation.
In the epithelium, proliferative growth correlated with downreulation of tumor suppressor Nkx3-1. Thsi genes is positively regulated by the androgen receptor; in inlfamed prostates, androgen receptor activation was decreased, offering a potential explanation for hyperplastic changes.
See our publication in Neoplasia for details.