Body on a Chip: Difference between revisions
| (46 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{Template:CHEM-ENG590E}} | {{Template:CHEM-ENG590E}} | ||
==Introduction== | |||
Body-on-a-chip, [https://openwetware.org/wiki/Organ-on-a-chip_-_Dan_Nguyen Organ-on-a-chip], and Tumor-on-a-chip all represent a consolidation of microfluidic technologies and biological practices in efforts to model body processes on a higher level of detail and complexity. Through the development of Lab-on-a-chip devices, these microfluidic devices have opened a door that could allow for more human-specific research to occur. | |||
Microfluidic technology offers a number of engineering advantages that make organ-on-a-chip systems versatile and useful. These devices often have external dimensions less than an inch and generally contain channels that have at least one dimension that is less than 1 mm. The small size of microfluidic devices results in a need for less reagents and experiment time than traditional systems.[1] Additionally, these lengths scales typically produce fluidic systems with Reynold’s numbers in the 1-100 range. This results in the creation of extremely controllable laminar flow, which can be used to impart precise physical forces, such as wall shear stress, cyclic strain, tensile and compressive forces on cells when used in organ-on-a-chip applications.[2] This is advantageous when trying to mimic the function of an organ by producing physiologically relevant mechanical forces that trigger a biological response. Microfluidic organ-on-a-chip systems can be designed with detailed control of experimental parameters, especially through the construction of mechanical and chemical gradients.[2] Additionally, microfluidics can be used to model specific diseases such as cancer. Using microfluidics, the effect of biochemical gradients and other non-cancerous components of the tumor microenvironment on cancer proliferation can be studied. Microfluidics also promises to be a powerful platform for in vitro pharmacokinetic studies of cancer therapeutics by allowing for drug transport properties to be studied in a 3D setting more akin to in vivo conditions. | |||
=== Limitations of Current Preclinical Techniques === | === Limitations of Current Preclinical Techniques === | ||
Modern advancements in molecular biology and genetics have allowed for the identification of an abundance of novel drug targets. However, many of these targets fail phase II and III clinical trials due to a lack of clinical efficacy. One of the major contributions to the ever-increasing failure rates is the dependency on in vitro (cell culture) and in vivo (animal experiments) as a means to effectively judge early efficacy, toxicity, and | Modern advancements in molecular biology and genetics have allowed for the identification of an abundance of novel drug targets. However, many of these targets fail phase II and III clinical trials due to a lack of clinical efficacy. One of the major contributions to the ever-increasing failure rates is the dependency on in vitro (cell culture) and in vivo (animal experiments) as a means to effectively judge early efficacy, toxicity, and pharmacokinetics.[3] Animal models that are executed well provide a reasonable means to test efficacy, but less than 8% of all cancer drug trials in animals end up having a successful transition to human trials.[3] Animals, predominantly rodents such as mice, are commonly used as a means to simulate human disease. However, mice models of cancer (amongst many other diseases) fail on several levels, with issues ranging from a genetic level, where 41% to 81% of over 4,000 shared genes had differing binding sites [4], to only being able to replicate a set of disease pathologies without including the entire spectrum of physiological changes that occur in humans12. Thus, there was a need to introduce a method of replicating human processes that was ethical, replicable, and precise. | ||
== Engineering Advantages and Limitations == | == Engineering Advantages and Limitations == | ||
| Line 7: | Line 13: | ||
=== Conventional Systems === | === Conventional Systems === | ||
The development of traditional three-dimensional (3D) cell culture systems has expanded the capabilities to model physiological environments in vitro, elucidating the function of organs, tissues, and diseases. Conventional systems utilize hydrogels, tissue-engineered constructs, and macroscale bioreactors to perform mechanical, biological, and chemical | The development of traditional three-dimensional (3D) cell culture systems has expanded the capabilities to model physiological environments in vitro, elucidating the function of organs, tissues, and diseases. Conventional systems utilize hydrogels, tissue-engineered constructs, and macroscale bioreactors to perform mechanical, biological, and chemical processes [2]. Many studies are conducted using organoids, miniature and simplified organs, which model the mammary gland[5], intestines[6], and brain[7], among others. Organoids can be constructed by inducing clustering of polarized organ-specific cells which interact with each other in the ECM of a hydrogel, for example.[2] The impact of contractile modifiers on micro-cardiac muscle tissue has been analyzed[8,9], as well as models of heart failure and cardiomyopathy [8,9]. The 3D nature of conventional 3D culture systems has permitted these developments and offers several other advantages. | ||
The physiological structure of various organs partially facilitates their function, which often can be only be modeled by 3D systems. Examples include the cognitive ability of the brain and the mechanical responses of bones, ligaments, and tendons[2]. The use of traditional organoids and tissue sections allows for recreation of the spatial heterogeneity which is present in the body, including the diverse tissue interactions in the lungs [2]. Drug performance can also be elucidated using macro culture chambers or bioreactors, which can be fluidically connected to model interactions between organoids [2]. Bioreactors and tissue-engineered constructs can also be advantageous when large samples are desired for analytical protocols such as mass spectroscopy [2]. However, the simplicity of many conventional systems limits their potential to understand the complex function of physiological milieus. | |||
[[Image:OrganoidPic.png|thumb|center|600px|[http://scienceblogs.com/pharyngula/files/2013/08/organoid_method.png]Figure 1: Example of traditional cerebral organoid tissue culture in a macro bioreactor.]] | |||
=== | === Body-on-a-chip (integration of multiple organ-on-a-chips) Advantages === | ||
The introduction of organs-on-a-chip | The introduction of organs-on-a-chip and combining multiple organ-on-a-chips have revolutionized the field of physiological modeling in vitro, predominantly because these microsystems allow for precise control over several experimental variables simultaneously. For example, the combination of specific cell types and their location relative to other cell types elucidates how different kinds of tissue interact [2]. Not only this, but controlling the position of experimental elements, such as cell cultures, makes it much easier to integrate visualization technology, including fluorescence microscopy, microflourimetry, and analytical assays than traditional systems [2]. Precise control over the organ-on-a-chip microstructure is also beneficial when developing computational fluid dynamic (CFD) models, which may be used to study the function of cells, metabolites, and gases based on their CFD behaviors [2]. | ||
One crucial advantage to using | One crucial advantage to using body-on-a-chips arises from the ability to control fluid flow in these systems. This is important because fluid interactions are prevalent in the body and affect the function and endurance of many cell types. By using organs-on-a-chips, cell cultures can be sustained for extended periods of time; human lung cells have been sustained in culture on a microfluidic chip system for over a month [10]. Cells respond to mechanical and chemical stimuli. Flow in microsystems engineering allows for easy establishment of mechanical and chemical gradients to determine how cells respond to fluid forces, cytokines, hormones, and gases[2]. If the cells being analyzed circulate physiologically, such as circulating tumor cells or bacteria, flow allows for a more accurate model of what is expected of these cells’ functions in vivo[12]. Not only do microsystems help accurately replicate cell, tissue, and organ function through the precise control of various parameters, but they can also produce larger sample sizes than conventional systems, allowing for more statistically significant experiments[2]. This is especially useful for performing drug testing on organs-on-a-chip. | ||
[[Image:OrganOnAChipPic.jpg|thumb|center|450px|[https://cdn.thenewstack.io/media/2015/04/organs-on-a-chip-wyss-institute-3.jpg]Figure 2: Concept of performing drug testing using organ-a-chip-technology.]] | |||
=== Body-on-a-chip (integration of multiple organ-on-a-chips) Limitations === | |||
Although the benefits of microsystems are prominent and still expanding, there are a number of technical challenges that must be overcome to advance the technology. Fabricating microfluidic chambers is a well-understood process. However, it usually requires advanced microfabrication technology, which can be expensive. Common fabrication issues, such as bubble formation within microstructures, can be detrimental to the survival of cells and the function of the microsystem.[2] When culturing cells in microchannels, seeding consistency is an issue, as is contamination100. Furthermore, once seeded, it is often difficult to ensure that cells will interact with each other and with a synthesized matrix in the same way that they would in vivo.[2] | |||
Given these challenges, microfluidic technology still offers enormous potential toward elucidating the role of physiological environments in vitro. The next step in evolving this technology will require the junction of functional units of organs to recreate entire organ systems, which can then be joined by synthetic microvessels coated with endothelial monolayers to form a more complete replica of the human body, which has coined the phrase “body-on-a-chip” 100. Commercially, this will have a huge effect on drug testing trials. By simulating the physiological environment and providing statistically significant experiments, organ-on-a-chip technology may be used instead of animal or human trials to evaluate drug performance. | |||
== Body-on-a-chip == | |||
Body-on-a-chip devices are the integration of multiple/different organ-on-a-chips that provide a unique opportunity to replicate complex, human-level organization. These microfluidic devices allow for the mimicking of organ-specific 3D architectures and exquisitely precise control of enabling fluid flow, nutrient and growth factor supply, carbon dioxide and oxygen concentrations, as well as adequate waste removal, in a human-level. Body on a chips are best suited to study pathophysiologies that are largely related to 3D microarchitecture and perfusion, as well as modeling acute disease (<1month timeframe). [11] | |||
This control of environmental factors allow for cells to not only survive in culture for longer, but also allow for an ability to introduce experimental factors such as drugs and disease in an environment that more closely mimics human organ function. Currently, these devices are best suited to study pathophysiologies that are largely related to 3D microarchitecture and perfusion, as well as modeling acute disease (<1 month timeframe). [11] By utilizing biomimetics, biotechnologists and chemical engineers have been able to replicate the cell architectures of: liver, kidney, intestine, lung, heart, smooth and striated muscle, fat, bone, marrow, cornea, skin, blood vessels, nerves, and the blood-brain barrier. [11] | |||
One of the major drawbacks of organ-on-a-chip devices is that they are generally not a good example of overall organ function, as there is a limited variety of the types of cells included. However, the relative heterogeneity of cell types allow for a more specific analysis of the physical effects of flow and shear, as well as cell-type-specific interactions with organ function, disease, and drug toxicity. This allows for more accurate in vitro predictions of the pathophysiological mechanisms of organ function, disease, and drug efficacy and toxicity screening before human trials. | One of the major drawbacks of organ-on-a-chip devices is that they are generally not a good example of overall organ function, as there is a limited variety of the types of cells included. However, the relative heterogeneity of cell types allow for a more specific analysis of the physical effects of flow and shear, as well as cell-type-specific interactions with organ function, disease, and drug toxicity. This allows for more accurate in vitro predictions of the pathophysiological mechanisms of organ function, disease, and drug efficacy and toxicity screening before human trials. | ||
[[Image:bodychipLD.gif|thumb|center|600px|[http://www.thelatestnews.com/organ-chip-breakthrough-replace-lab-animals/]Figure | [[Image:bodychipLD.gif|thumb|center|600px|[http://www.thelatestnews.com/organ-chip-breakthrough-replace-lab-animals/]Figure 3: Examples of some of the basic structures of three different organ-on-a-chip devices.]] | ||
=== Liver-on-a-Chip === | === Liver-on-a-Chip === | ||
[[Image:liverchipLD.png|thumb|right|200px|[https://ncats.nih.gov/tissuechip/chip/liver]Figure | [[Image:liverchipLD.png|thumb|right|200px|[https://ncats.nih.gov/tissuechip/chip/liver]Figure 4: One example of a liver-on-a-chip using iPSC-HPs and a synthetic endothelium layer to replicate ''in vivo'' structure]] | ||
One of the best examples of utilizing organ-on-a-chip devices to study drug efficacy and toxicity is the | One of the best examples of utilizing organ-on-a-chip devices to study drug efficacy and toxicity in a human body is the liver-on-a-Chip. The liver is especially sensitive to drug-induced injury, and liver toxicity is one of the leading reasons for discontinuing clinical trials, and is the cause of a majority of costly late stage drug trial failures [12, 15]. Previous in vitro studies struggled to adequately replicate the organ environment, as the general strata of the liver is described as areas of high homotypic cell density with a complex apico-basal polarity that allows for the rise of the bile canaliculi (BC) network [14], as well as a prolific sinusoidal blood vessel network. While the BC vessel network development is mainly supported by the 3D configuration of hepatocytes, the basic structure of the sinusoidal vessels consist of endothelial cells and occasional Kupffer (immune) cells. Thus, when creating the Liver-on-a-Chip, one of the highest priorities is to address and support the 3D structure of hepatocytes and their intimate relationship with sinusoidal endothelial cells [13]. As seen in fig. 1, the iPSC-HPs (induced pluripotent stem cells - hepatocytes) are separated from the medium by an endothelium-like barrier, and cell-cell interactions allowed for this particular device to maintain viability for over four weeks [14]. Physiological markers of cell function (e.g.- Albumin, AST, ALT, etc.) were used as a benchmarks to measure health and survivability of the cells [15], and the deviations in these results were used as a method to determine drug uptake and subsequent cell death in studies investigating drug-specific hepatotoxicity202. | ||
=== Lung-on-a-Chip === | === Lung-on-a-Chip === | ||
[[Image:lungchipLD.jpg|thumb|left|500px|[http://www.thelatestnews.com/organ-chip-breakthrough-replace-lab-animals/]Figure | [[Image:lungchipLD.jpg|thumb|left|500px|[http://www.thelatestnews.com/organ-chip-breakthrough-replace-lab-animals/]Figure 5: Design of a flexible Lung-on-a-Chip]] | ||
While the Liver-on-a-Chip was an excellent device to test drug toxicity using human hepatocytes, the 3D structure remains relatively straightforward. An example of using organ-on-a-chip to more effectively mimic and study 3D structure in a way that was previously inaccessible is the Lung-on-a-Chip. The fundamental functional units of the lung are the alveolar cells and the complex and intimately involved capillary network, but efforts to replicate this process in vitro struggle to capture the mechanical movement implicit in lung | While the Liver-on-a-Chip was an excellent device to test drug toxicity using human hepatocytes, the 3D structure remains relatively straightforward. An example of using organ-on-a-chip to more effectively mimic and study 3D structure in a way that was previously inaccessible is the Lung-on-a-Chip. The fundamental functional units of the lung are the alveolar cells and the complex and intimately involved capillary network, but efforts to replicate this process in vitro struggle to capture the mechanical movement implicit in lung function [19]. In vitro studies also struggled to replicate the alveolar-capillary interface, as it required growth of cells on both sides of a membrane. With the use of a microfluidic device that utilized a flexible membrane and a vacuum with flanking chambers as seen in fig. 2208, Huh et al. was able to replicate movement and structure that closely resembled that of a human lung. The device, like many others like it, consisted of a porous, flexible membrane that had alveolar epithelial cells grown on one side and pulmonary microvascular endothelial cells on the opposite side[20]. Using a device that replicated the flex-relax cycle of breathing lead to a number of kinetic-influenced discoveries, such as in one trial there was a several-fold increase in the uptake of silica nanoparticles than the conventional methods of cell culture described. In another Lung-on-a-Chip experiment, this time studying the cancer drug interleukin-2 (IL-2) and the pulmonary edema that occurs when high levels of IL-2 are administered, it was discovered that purely mechanical forces contributed significantly to the development of vascular leakage and subsequent pulmonary edema[21]. Through creating a device that was able to replicate the structure and movement, developments surrounding previously-unknown factors are now coming to light, which will further influence the way medicine will approach the development of lung disease and the role movement plays. | ||
=== Kidney-on-a-Chip === | === Kidney-on-a-Chip === | ||
[[Image:kidneychipLD.gif|thumb|lright|500px|[http://www.thelatestnews.com/organ-chip-breakthrough-replace-lab-animals/]Figure | [[Image:kidneychipLD.gif|thumb|lright|500px|[http://www.thelatestnews.com/organ-chip-breakthrough-replace-lab-animals/]Figure 6: Examples of some of the basic structures of three different organ-on-a-chip devices.]] | ||
Kidney-on-a-Chip effectively combines the leading motivations behind the developments of Lung- and Liver-on-a-Chip, as the kidney faces both structural 3D microenvironments and issues with drug-induced renal toxicity and injury. Unfortunately, advancements in creating an effective and relevant Kidney-on-a-Chip are hindered by the cellular composition and 3D architecture of the kidney, as it presents a fairly significant translational barrier. There are more than ten renal cell | Kidney-on-a-Chip effectively combines the leading motivations behind the developments of Lung- and Liver-on-a-Chip, as the kidney faces both structural 3D microenvironments and issues with drug-induced renal toxicity and injury. Unfortunately, advancements in creating an effective and relevant Kidney-on-a-Chip are hindered by the cellular composition and 3D architecture of the kidney, as it presents a fairly significant translational barrier. There are more than ten renal cell types[21] arranged in an increasingly complex 3D structure, which is further compounded by a complex vasculature system. Even the main function of the organ presents as a complex issue, as there is filtration by the glomeruli and a two-way active secretion and reabsorption process in the tubular apparatus, all occurring in tandem[22]. However, these uptake processes are central in understanding the mechanism of drug-induced renal toxicity and quantifying sensitivity to toxic agents[22]. Another issue that plagues kidneys-on-a-chip is the dependence on nonhuman cell lines such as the Madin-Darby canine kidney (MDCK) cell line as cellular basis for the chips, as immortal human cell lines struggle to express central transporter families and are transitioning away from epithelial cells to mesenchymal cells[23]. Freshly derived human renal cells show promise, but there are issues with donor variability and maintaining their specificity as time goes on[23]. | ||
Nonetheless, chips have been developed using a multi-level flow through design, as seen in fig. 3304. The basic structure of the proximal tubule is replicated by sandwiching a layer of human proximal tubular epithelial cells on an ECM-coated porous membrane, then exposing both sides of the membrane to constant flow. | Nonetheless, chips have been developed using a multi-level flow through design, as seen in fig. 3304. The basic structure of the proximal tubule is replicated by sandwiching a layer of human proximal tubular epithelial cells on an ECM-coated porous membrane, then exposing both sides of the membrane to constant flow. | ||
| Line 50: | Line 61: | ||
Modeling Biochemical Regulation of Cancer Proliferation | Modeling Biochemical Regulation of Cancer Proliferation | ||
The growth, differentiation, and migration of tumor cells are regulated by the biochemical and biophysical properties of the tumor microenvironment. Many of these processes are controlled through various biochemical gradients which can be modeled using microfluidics. There are two main methods by which this is accomplished: flow-based methods and diffusion based methods. The former utilizes convective forces with in laminar flow regimes to produce chemical gradients, while the latter uses passive diffusion of molecules to form gradients201. For example, multiple studies have been conducted on the effects of Epidermal Growth Factor (EGF) gradients on cancer cell | The growth, differentiation, and migration of tumor cells are regulated by the biochemical and biophysical properties of the tumor microenvironment. Many of these processes are controlled through various biochemical gradients which can be modeled using microfluidics. There are two main methods by which this is accomplished: flow-based methods and diffusion based methods. The former utilizes convective forces with in laminar flow regimes to produce chemical gradients, while the latter uses passive diffusion of molecules to form gradients201. For example, multiple studies have been conducted on the effects of Epidermal Growth Factor (EGF) gradients on cancer cell chemotaxis[24] (Fig. 1) | ||
[[Image:flow-based gradient microchannel.png|thumb|left|500px|Figure 7: A flow-based gradient generation microchannel network for examining the effect of EGF gradients on breast cancer chemotaxis]] | |||
Modeling Non-Cancerous Cell Components of the Tumor Microenvironment | Modeling Non-Cancerous Cell Components of the Tumor Microenvironment | ||
Fibroblasts are connective tissue cells found to be associated with all stages of cancer proliferation. Fibroblasts are recruited by tumor cells to produce specific growth factors. Microfluidics have been used to simulate these interactions in vitro. For example, Qin et al. fabricated a multichambered device for the co-culture of fibroblasts and tumor cells while allowing for diffusional interactions between parallel channels | Fibroblasts are connective tissue cells found to be associated with all stages of cancer proliferation. Fibroblasts are recruited by tumor cells to produce specific growth factors. Microfluidics have been used to simulate these interactions in vitro. For example, Qin et al. fabricated a multichambered device for the co-culture of fibroblasts and tumor cells while allowing for diffusional interactions between parallel channels [25] (Fig. 2). | ||
[[Image:fibroblast interaction.png|thumb|right|500px|Figure 8: Microfluidic device for the characterization of the interaction between fibroblasts ( in chambers) and tumor cells in medium. Fibroblasts exhibit activcation in the presence of cancer cell enriched medium as the stretched body and high expression of α-SMA (a fibroblast signaling molecule]] | |||
Figure | |||
=== Modeling Drug Delivery: Overview and Motivation === | === Modeling Drug Delivery: Overview and Motivation === | ||
Microfluidics can be used to model pharmacokinetics in a 3D environment. Microfluidics provide a promising alternative to monolayer or mouse experiments for predicting drug efficacy in vivo. Traditional monolayer experiments only examine cytotoxicity in a 2D environment. This ignores the transport properties of the drug, which can significantly impact drug efficacy. Upon intravenous delivery, the blood plasma concentration of a drug is at its highest value. The plasma concentration then decreases over time via absorption by cells and metabolism. The drug spreads through tumors via diffusion, and may bind to target receptors. Once the plasma concentration of a drug falls below the cellular concentration, the drug is cleared from the system. The cytotoxicity of a drug is effected by the combination of these transport processes. | Microfluidics can be used to model pharmacokinetics in a 3D environment. Microfluidics provide a promising alternative to monolayer or mouse experiments for predicting drug efficacy in vivo. Traditional monolayer experiments only examine cytotoxicity in a 2D environment. This ignores the transport properties of the drug, which can significantly impact drug efficacy. Upon intravenous delivery, the blood plasma concentration of a drug is at its highest value. The plasma concentration then decreases over time via absorption by cells and metabolism. The drug spreads through tumors via diffusion, and may bind to target receptors. Once the plasma concentration of a drug falls below the cellular concentration, the drug is cleared from the system. The cytotoxicity of a drug is effected by the combination of these transport processes.[26] Drugs that demonstrate cytotoxicity in monolayer are generally then tested in mice. However, there is generally no correlation between efficacy in monolayer and efficacy in mice. Moreover, experiments in mice are expensive and time consuming. Using microfluidics, the transport of a therapy from the blood vessel into tumors can be simulated. [26] (Fig.3) | ||
Comparing Transport Properties of Doxirubicin and Doxil on Microfluidic Device | Comparing Transport Properties of Doxirubicin and Doxil on Microfluidic Device | ||
Such a device was created by the Forbes lab at UMass Amherst. The dimensions of the device were 1000 mm x 300 mm x 150 mm, and a microfluidic channel, 250 mm wide and 150 mm tall (Fig. 4). Experiments were conducted using human colon carcinoma spheroids. The device was designed to mimic the interface between a tumor and surrounding | Such a device was created by the Forbes lab at UMass Amherst. The dimensions of the device were 1000 mm x 300 mm x 150 mm, and a microfluidic channel, 250 mm wide and 150 mm tall (Fig. 4). Experiments were conducted using human colon carcinoma spheroids. The device was designed to mimic the interface between a tumor and surrounding vasculature [26]. | ||
Figure | [[Image:drug transport.png|thumb|left|300px|Figure 9: Mechanisms determining therapeutic efficacy. (A) Intravenously injected chemotherapeutic drugs enter tumors by diffusion. (B) Drug binding. (C) Drug clearance from tumor tissue. (D–F)]] | ||
[[Image:microfluidic tumor-on-a-chip.png|thumb|right|300px|Figure 10: Drug delivery device constructed from Polydimethylsiloxane (PDMS). Drug is delivered to the tumor tissue via microfluidic channel, and observed under a microscope]] | |||
[[Image:dox results.png|thumb|right|300px|Figure 11: (A,B) Uptake and Clearance progression for Doxil and Doxirubicin respectively, (C,D, E) Concentration vs. Time profiles for each drug. Doxorubicin out performs Doxil through longer clearance times]] | |||
Tumor spheroids in the device were treated using Doxirubicin and Doxil. Each drug was delivered with a green fluorescent stain to help track uptake. The accumulation of the drug within the spheroids positively correlated with apoptosis (cell death). In the case of doxorubicin, it was observed that the drug has a slow clearance rate (20 hrs) and a corresponding higher extent of apoptosis than doxil, which cleared in only 7hrs [26]. (Fig. 5) | |||
== Future Work and Ultimate Goals== | |||
Microfluidic devices have the potential to serve as analogs for organs in vitro. As the technology matures further and human in vitro tissue culture becomes easier, full organ functions could be modeled in devices. This would create a platform for an in depth analysis of disease mechanisms as well as the power of new therapies. By integrating these microfluidic organs, entire organ systems or even a complete body could be modeled in vitro on a microscale. This would allow for an unprecedented ability to examine the effects of diseases and drugs across the entire human body. | |||
== References == | == References == | ||
[1] Amin, R. et al. 3-D Printed Microfluidic devices. Biofabrication. 8 022001 (2016). | |||
[ | [2] Bhatia S. N. & Ingber D. E. Microfluidic organs-on-chips. Nature biotechnology 32, 760–772, doi: (2014).10.1038/nbt.2989. | ||
[ | [3] Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. American Journal of Translational Research. 2014;6(2):114-118. | ||
[ | [4] Gawrylewski A. The Trouble with Animal Models. The Scientist. 2007 | ||
[ | [5] Fingleton B. Matrix metalloproteinases as valid clinical targets. Curr Pharm Des. 2007;13:333–46. | ||
[ | [6] Mroue, R. & Bissell, M.J. Three-dimensional cultures of mouse mammary epithelial cells. Methods Mol. Biol. 945, 221–250 (2013). | ||
cells. Methods Mol. Biol. 945, 221–250 (2013). | |||
[ | [7] Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanisms and applications. Science 340, 1190–1194 (2013). | ||
cell: mechanisms and applications. Science 340, 1190–1194 (2013). | |||
[ | [8] Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). | ||
[ | [9] Grosberg, A., Alford, P.W., McCain, M.L. & Parker, K.K. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 11, 4165–4173 (2011). | ||
cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip | |||
11, 4165–4173 (2011). | |||
[ | [10] Boudou, T. et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 18, 910–919 (2012). | ||
of engineered cardiac microtissues. Tissue Eng. Part A 18, 910–919 (2012). | |||
[ | [11] McCain, M.L., Sheehy, S.P., Grosberg, A., Goss, J.A. & Parker, K.K. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl. Acad. Sci. USA 110, 9770–9775 (2013). | ||
maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl. Acad. | |||
Sci. USA 110, 9770–9775 (2013). | |||
[ | [12] Wang, G. et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623 (2014). | ||
induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623 | |||
(2014). | |||
[ | [13] Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010). | ||
1662–1668 (2010). | |||
[ | [14] Kim, H.J., Huh, D., Hamilton, G. & Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174 (2012). | ||
microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip | |||
12, 2165–2174 (2012). | [15] H.Y. Tiong, et al. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol. Pharm., 11 (2014), pp. 1933–1948 | ||
[ | |||
[ | [16] Wilmer, Martijn J., et al. "Kidney-on-a-chip technology for drug-induced nephrotoxicity screening." Trends in biotechnology 34.2 (2016): 156-170. | ||
[ | [17] Van der Hauwaert, Cynthia, et al. "Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling." PLoS One 8.6 (2013): e66750. | ||
[ | [18] Jang, Kyung-Jin, et al. "Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment." Integrative Biology 5.9 (2013): 1119-1129. | ||
[ | [19] Wang S. J., Saadi W., Lin F., Nguyen C. M. C., and Jeon N. L., Exp. Cell Res. 300, 180 (2004) | ||
[ | [20] Hare, P. (2010). Lung on a chip. Nature Biotechnology, 28(8), 816-817. | ||
[ | [21] Esch, E. W., Bahinski, A., & Huh, D. (2015). Organs-on-chips at the frontiers of drug discovery. Nature reviews Drug discovery, 14(4), 248-260. | ||
[ | [22] Bhatia, S. N., & Ingber, D. E. (2014). Microfluidic organs-on-chips. Nature biotechnology, 32(8), 760-772. | ||
[ | [23] Huh, D., Leslie, D. C., Matthews, B. D., Fraser, J. P., Jurek, S., Hamilton, G. A., ... & Ingber, D. E. (2012). A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Science translational medicine, 4(159), 159ra147-159ra147. | ||
[ | [24] Ma H., Xu H. & Qin J. Biomimetic tumor microenvironment on a microfluidic platform. Biomicrofluidics. 7, 11501 (2013). | ||
[ | [25] Liu T., Lin B., and Qin J., Lab Chip 10, 1671 (2010). | ||
[ | [26] Toley, B. J., Ganz, D. E., Walsh, C. L. & Forbes, N. S. Microfluidic device for recreating a tumor microenvironment in vitro. J. Vis. Exp. 5–9 (2011). | ||
Latest revision as of 18:31, 19 April 2020
Introduction
Body-on-a-chip, Organ-on-a-chip, and Tumor-on-a-chip all represent a consolidation of microfluidic technologies and biological practices in efforts to model body processes on a higher level of detail and complexity. Through the development of Lab-on-a-chip devices, these microfluidic devices have opened a door that could allow for more human-specific research to occur.
Microfluidic technology offers a number of engineering advantages that make organ-on-a-chip systems versatile and useful. These devices often have external dimensions less than an inch and generally contain channels that have at least one dimension that is less than 1 mm. The small size of microfluidic devices results in a need for less reagents and experiment time than traditional systems.[1] Additionally, these lengths scales typically produce fluidic systems with Reynold’s numbers in the 1-100 range. This results in the creation of extremely controllable laminar flow, which can be used to impart precise physical forces, such as wall shear stress, cyclic strain, tensile and compressive forces on cells when used in organ-on-a-chip applications.[2] This is advantageous when trying to mimic the function of an organ by producing physiologically relevant mechanical forces that trigger a biological response. Microfluidic organ-on-a-chip systems can be designed with detailed control of experimental parameters, especially through the construction of mechanical and chemical gradients.[2] Additionally, microfluidics can be used to model specific diseases such as cancer. Using microfluidics, the effect of biochemical gradients and other non-cancerous components of the tumor microenvironment on cancer proliferation can be studied. Microfluidics also promises to be a powerful platform for in vitro pharmacokinetic studies of cancer therapeutics by allowing for drug transport properties to be studied in a 3D setting more akin to in vivo conditions.
Limitations of Current Preclinical Techniques
Modern advancements in molecular biology and genetics have allowed for the identification of an abundance of novel drug targets. However, many of these targets fail phase II and III clinical trials due to a lack of clinical efficacy. One of the major contributions to the ever-increasing failure rates is the dependency on in vitro (cell culture) and in vivo (animal experiments) as a means to effectively judge early efficacy, toxicity, and pharmacokinetics.[3] Animal models that are executed well provide a reasonable means to test efficacy, but less than 8% of all cancer drug trials in animals end up having a successful transition to human trials.[3] Animals, predominantly rodents such as mice, are commonly used as a means to simulate human disease. However, mice models of cancer (amongst many other diseases) fail on several levels, with issues ranging from a genetic level, where 41% to 81% of over 4,000 shared genes had differing binding sites [4], to only being able to replicate a set of disease pathologies without including the entire spectrum of physiological changes that occur in humans12. Thus, there was a need to introduce a method of replicating human processes that was ethical, replicable, and precise.
Engineering Advantages and Limitations
Conventional Systems
The development of traditional three-dimensional (3D) cell culture systems has expanded the capabilities to model physiological environments in vitro, elucidating the function of organs, tissues, and diseases. Conventional systems utilize hydrogels, tissue-engineered constructs, and macroscale bioreactors to perform mechanical, biological, and chemical processes [2]. Many studies are conducted using organoids, miniature and simplified organs, which model the mammary gland[5], intestines[6], and brain[7], among others. Organoids can be constructed by inducing clustering of polarized organ-specific cells which interact with each other in the ECM of a hydrogel, for example.[2] The impact of contractile modifiers on micro-cardiac muscle tissue has been analyzed[8,9], as well as models of heart failure and cardiomyopathy [8,9]. The 3D nature of conventional 3D culture systems has permitted these developments and offers several other advantages.
The physiological structure of various organs partially facilitates their function, which often can be only be modeled by 3D systems. Examples include the cognitive ability of the brain and the mechanical responses of bones, ligaments, and tendons[2]. The use of traditional organoids and tissue sections allows for recreation of the spatial heterogeneity which is present in the body, including the diverse tissue interactions in the lungs [2]. Drug performance can also be elucidated using macro culture chambers or bioreactors, which can be fluidically connected to model interactions between organoids [2]. Bioreactors and tissue-engineered constructs can also be advantageous when large samples are desired for analytical protocols such as mass spectroscopy [2]. However, the simplicity of many conventional systems limits their potential to understand the complex function of physiological milieus.

Body-on-a-chip (integration of multiple organ-on-a-chips) Advantages
The introduction of organs-on-a-chip and combining multiple organ-on-a-chips have revolutionized the field of physiological modeling in vitro, predominantly because these microsystems allow for precise control over several experimental variables simultaneously. For example, the combination of specific cell types and their location relative to other cell types elucidates how different kinds of tissue interact [2]. Not only this, but controlling the position of experimental elements, such as cell cultures, makes it much easier to integrate visualization technology, including fluorescence microscopy, microflourimetry, and analytical assays than traditional systems [2]. Precise control over the organ-on-a-chip microstructure is also beneficial when developing computational fluid dynamic (CFD) models, which may be used to study the function of cells, metabolites, and gases based on their CFD behaviors [2].
One crucial advantage to using body-on-a-chips arises from the ability to control fluid flow in these systems. This is important because fluid interactions are prevalent in the body and affect the function and endurance of many cell types. By using organs-on-a-chips, cell cultures can be sustained for extended periods of time; human lung cells have been sustained in culture on a microfluidic chip system for over a month [10]. Cells respond to mechanical and chemical stimuli. Flow in microsystems engineering allows for easy establishment of mechanical and chemical gradients to determine how cells respond to fluid forces, cytokines, hormones, and gases[2]. If the cells being analyzed circulate physiologically, such as circulating tumor cells or bacteria, flow allows for a more accurate model of what is expected of these cells’ functions in vivo[12]. Not only do microsystems help accurately replicate cell, tissue, and organ function through the precise control of various parameters, but they can also produce larger sample sizes than conventional systems, allowing for more statistically significant experiments[2]. This is especially useful for performing drug testing on organs-on-a-chip.

Body-on-a-chip (integration of multiple organ-on-a-chips) Limitations
Although the benefits of microsystems are prominent and still expanding, there are a number of technical challenges that must be overcome to advance the technology. Fabricating microfluidic chambers is a well-understood process. However, it usually requires advanced microfabrication technology, which can be expensive. Common fabrication issues, such as bubble formation within microstructures, can be detrimental to the survival of cells and the function of the microsystem.[2] When culturing cells in microchannels, seeding consistency is an issue, as is contamination100. Furthermore, once seeded, it is often difficult to ensure that cells will interact with each other and with a synthesized matrix in the same way that they would in vivo.[2]
Given these challenges, microfluidic technology still offers enormous potential toward elucidating the role of physiological environments in vitro. The next step in evolving this technology will require the junction of functional units of organs to recreate entire organ systems, which can then be joined by synthetic microvessels coated with endothelial monolayers to form a more complete replica of the human body, which has coined the phrase “body-on-a-chip” 100. Commercially, this will have a huge effect on drug testing trials. By simulating the physiological environment and providing statistically significant experiments, organ-on-a-chip technology may be used instead of animal or human trials to evaluate drug performance.
Body-on-a-chip
Body-on-a-chip devices are the integration of multiple/different organ-on-a-chips that provide a unique opportunity to replicate complex, human-level organization. These microfluidic devices allow for the mimicking of organ-specific 3D architectures and exquisitely precise control of enabling fluid flow, nutrient and growth factor supply, carbon dioxide and oxygen concentrations, as well as adequate waste removal, in a human-level. Body on a chips are best suited to study pathophysiologies that are largely related to 3D microarchitecture and perfusion, as well as modeling acute disease (<1month timeframe). [11] This control of environmental factors allow for cells to not only survive in culture for longer, but also allow for an ability to introduce experimental factors such as drugs and disease in an environment that more closely mimics human organ function. Currently, these devices are best suited to study pathophysiologies that are largely related to 3D microarchitecture and perfusion, as well as modeling acute disease (<1 month timeframe). [11] By utilizing biomimetics, biotechnologists and chemical engineers have been able to replicate the cell architectures of: liver, kidney, intestine, lung, heart, smooth and striated muscle, fat, bone, marrow, cornea, skin, blood vessels, nerves, and the blood-brain barrier. [11] One of the major drawbacks of organ-on-a-chip devices is that they are generally not a good example of overall organ function, as there is a limited variety of the types of cells included. However, the relative heterogeneity of cell types allow for a more specific analysis of the physical effects of flow and shear, as well as cell-type-specific interactions with organ function, disease, and drug toxicity. This allows for more accurate in vitro predictions of the pathophysiological mechanisms of organ function, disease, and drug efficacy and toxicity screening before human trials.

Liver-on-a-Chip

One of the best examples of utilizing organ-on-a-chip devices to study drug efficacy and toxicity in a human body is the liver-on-a-Chip. The liver is especially sensitive to drug-induced injury, and liver toxicity is one of the leading reasons for discontinuing clinical trials, and is the cause of a majority of costly late stage drug trial failures [12, 15]. Previous in vitro studies struggled to adequately replicate the organ environment, as the general strata of the liver is described as areas of high homotypic cell density with a complex apico-basal polarity that allows for the rise of the bile canaliculi (BC) network [14], as well as a prolific sinusoidal blood vessel network. While the BC vessel network development is mainly supported by the 3D configuration of hepatocytes, the basic structure of the sinusoidal vessels consist of endothelial cells and occasional Kupffer (immune) cells. Thus, when creating the Liver-on-a-Chip, one of the highest priorities is to address and support the 3D structure of hepatocytes and their intimate relationship with sinusoidal endothelial cells [13]. As seen in fig. 1, the iPSC-HPs (induced pluripotent stem cells - hepatocytes) are separated from the medium by an endothelium-like barrier, and cell-cell interactions allowed for this particular device to maintain viability for over four weeks [14]. Physiological markers of cell function (e.g.- Albumin, AST, ALT, etc.) were used as a benchmarks to measure health and survivability of the cells [15], and the deviations in these results were used as a method to determine drug uptake and subsequent cell death in studies investigating drug-specific hepatotoxicity202.
Lung-on-a-Chip
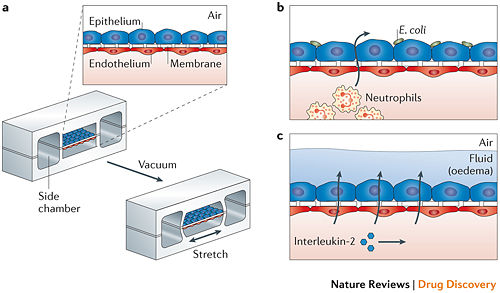
While the Liver-on-a-Chip was an excellent device to test drug toxicity using human hepatocytes, the 3D structure remains relatively straightforward. An example of using organ-on-a-chip to more effectively mimic and study 3D structure in a way that was previously inaccessible is the Lung-on-a-Chip. The fundamental functional units of the lung are the alveolar cells and the complex and intimately involved capillary network, but efforts to replicate this process in vitro struggle to capture the mechanical movement implicit in lung function [19]. In vitro studies also struggled to replicate the alveolar-capillary interface, as it required growth of cells on both sides of a membrane. With the use of a microfluidic device that utilized a flexible membrane and a vacuum with flanking chambers as seen in fig. 2208, Huh et al. was able to replicate movement and structure that closely resembled that of a human lung. The device, like many others like it, consisted of a porous, flexible membrane that had alveolar epithelial cells grown on one side and pulmonary microvascular endothelial cells on the opposite side[20]. Using a device that replicated the flex-relax cycle of breathing lead to a number of kinetic-influenced discoveries, such as in one trial there was a several-fold increase in the uptake of silica nanoparticles than the conventional methods of cell culture described. In another Lung-on-a-Chip experiment, this time studying the cancer drug interleukin-2 (IL-2) and the pulmonary edema that occurs when high levels of IL-2 are administered, it was discovered that purely mechanical forces contributed significantly to the development of vascular leakage and subsequent pulmonary edema[21]. Through creating a device that was able to replicate the structure and movement, developments surrounding previously-unknown factors are now coming to light, which will further influence the way medicine will approach the development of lung disease and the role movement plays.
Kidney-on-a-Chip

Kidney-on-a-Chip effectively combines the leading motivations behind the developments of Lung- and Liver-on-a-Chip, as the kidney faces both structural 3D microenvironments and issues with drug-induced renal toxicity and injury. Unfortunately, advancements in creating an effective and relevant Kidney-on-a-Chip are hindered by the cellular composition and 3D architecture of the kidney, as it presents a fairly significant translational barrier. There are more than ten renal cell types[21] arranged in an increasingly complex 3D structure, which is further compounded by a complex vasculature system. Even the main function of the organ presents as a complex issue, as there is filtration by the glomeruli and a two-way active secretion and reabsorption process in the tubular apparatus, all occurring in tandem[22]. However, these uptake processes are central in understanding the mechanism of drug-induced renal toxicity and quantifying sensitivity to toxic agents[22]. Another issue that plagues kidneys-on-a-chip is the dependence on nonhuman cell lines such as the Madin-Darby canine kidney (MDCK) cell line as cellular basis for the chips, as immortal human cell lines struggle to express central transporter families and are transitioning away from epithelial cells to mesenchymal cells[23]. Freshly derived human renal cells show promise, but there are issues with donor variability and maintaining their specificity as time goes on[23]. Nonetheless, chips have been developed using a multi-level flow through design, as seen in fig. 3304. The basic structure of the proximal tubule is replicated by sandwiching a layer of human proximal tubular epithelial cells on an ECM-coated porous membrane, then exposing both sides of the membrane to constant flow.
Kidney-on-a-Chip is a great example of how much room for growth the organ-on-a-chip field has. While the liver and lungs have a fairly homotypic cell type, the kidney has a significant increase in complexity and works as a great example for one of the major barriers that these microfluidic devices face.
Modeling Cancer: Tumor-on-a-Chip
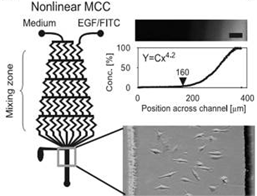
Modeling Biochemical Regulation of Cancer Proliferation The growth, differentiation, and migration of tumor cells are regulated by the biochemical and biophysical properties of the tumor microenvironment. Many of these processes are controlled through various biochemical gradients which can be modeled using microfluidics. There are two main methods by which this is accomplished: flow-based methods and diffusion based methods. The former utilizes convective forces with in laminar flow regimes to produce chemical gradients, while the latter uses passive diffusion of molecules to form gradients201. For example, multiple studies have been conducted on the effects of Epidermal Growth Factor (EGF) gradients on cancer cell chemotaxis[24] (Fig. 1)
Modeling Non-Cancerous Cell Components of the Tumor Microenvironment
Fibroblasts are connective tissue cells found to be associated with all stages of cancer proliferation. Fibroblasts are recruited by tumor cells to produce specific growth factors. Microfluidics have been used to simulate these interactions in vitro. For example, Qin et al. fabricated a multichambered device for the co-culture of fibroblasts and tumor cells while allowing for diffusional interactions between parallel channels [25] (Fig. 2).

Modeling Drug Delivery: Overview and Motivation
Microfluidics can be used to model pharmacokinetics in a 3D environment. Microfluidics provide a promising alternative to monolayer or mouse experiments for predicting drug efficacy in vivo. Traditional monolayer experiments only examine cytotoxicity in a 2D environment. This ignores the transport properties of the drug, which can significantly impact drug efficacy. Upon intravenous delivery, the blood plasma concentration of a drug is at its highest value. The plasma concentration then decreases over time via absorption by cells and metabolism. The drug spreads through tumors via diffusion, and may bind to target receptors. Once the plasma concentration of a drug falls below the cellular concentration, the drug is cleared from the system. The cytotoxicity of a drug is effected by the combination of these transport processes.[26] Drugs that demonstrate cytotoxicity in monolayer are generally then tested in mice. However, there is generally no correlation between efficacy in monolayer and efficacy in mice. Moreover, experiments in mice are expensive and time consuming. Using microfluidics, the transport of a therapy from the blood vessel into tumors can be simulated. [26] (Fig.3)
Comparing Transport Properties of Doxirubicin and Doxil on Microfluidic Device
Such a device was created by the Forbes lab at UMass Amherst. The dimensions of the device were 1000 mm x 300 mm x 150 mm, and a microfluidic channel, 250 mm wide and 150 mm tall (Fig. 4). Experiments were conducted using human colon carcinoma spheroids. The device was designed to mimic the interface between a tumor and surrounding vasculature [26].



Tumor spheroids in the device were treated using Doxirubicin and Doxil. Each drug was delivered with a green fluorescent stain to help track uptake. The accumulation of the drug within the spheroids positively correlated with apoptosis (cell death). In the case of doxorubicin, it was observed that the drug has a slow clearance rate (20 hrs) and a corresponding higher extent of apoptosis than doxil, which cleared in only 7hrs [26]. (Fig. 5)
Future Work and Ultimate Goals
Microfluidic devices have the potential to serve as analogs for organs in vitro. As the technology matures further and human in vitro tissue culture becomes easier, full organ functions could be modeled in devices. This would create a platform for an in depth analysis of disease mechanisms as well as the power of new therapies. By integrating these microfluidic organs, entire organ systems or even a complete body could be modeled in vitro on a microscale. This would allow for an unprecedented ability to examine the effects of diseases and drugs across the entire human body.
References
[1] Amin, R. et al. 3-D Printed Microfluidic devices. Biofabrication. 8 022001 (2016).
[2] Bhatia S. N. & Ingber D. E. Microfluidic organs-on-chips. Nature biotechnology 32, 760–772, doi: (2014).10.1038/nbt.2989.
[3] Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. American Journal of Translational Research. 2014;6(2):114-118.
[4] Gawrylewski A. The Trouble with Animal Models. The Scientist. 2007
[5] Fingleton B. Matrix metalloproteinases as valid clinical targets. Curr Pharm Des. 2007;13:333–46.
[6] Mroue, R. & Bissell, M.J. Three-dimensional cultures of mouse mammary epithelial cells. Methods Mol. Biol. 945, 221–250 (2013).
[7] Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanisms and applications. Science 340, 1190–1194 (2013).
[8] Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
[9] Grosberg, A., Alford, P.W., McCain, M.L. & Parker, K.K. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 11, 4165–4173 (2011).
[10] Boudou, T. et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 18, 910–919 (2012).
[11] McCain, M.L., Sheehy, S.P., Grosberg, A., Goss, J.A. & Parker, K.K. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl. Acad. Sci. USA 110, 9770–9775 (2013).
[12] Wang, G. et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623 (2014).
[13] Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010).
[14] Kim, H.J., Huh, D., Hamilton, G. & Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174 (2012).
[15] H.Y. Tiong, et al. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol. Pharm., 11 (2014), pp. 1933–1948
[16] Wilmer, Martijn J., et al. "Kidney-on-a-chip technology for drug-induced nephrotoxicity screening." Trends in biotechnology 34.2 (2016): 156-170.
[17] Van der Hauwaert, Cynthia, et al. "Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling." PLoS One 8.6 (2013): e66750.
[18] Jang, Kyung-Jin, et al. "Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment." Integrative Biology 5.9 (2013): 1119-1129.
[19] Wang S. J., Saadi W., Lin F., Nguyen C. M. C., and Jeon N. L., Exp. Cell Res. 300, 180 (2004)
[20] Hare, P. (2010). Lung on a chip. Nature Biotechnology, 28(8), 816-817.
[21] Esch, E. W., Bahinski, A., & Huh, D. (2015). Organs-on-chips at the frontiers of drug discovery. Nature reviews Drug discovery, 14(4), 248-260.
[22] Bhatia, S. N., & Ingber, D. E. (2014). Microfluidic organs-on-chips. Nature biotechnology, 32(8), 760-772.
[23] Huh, D., Leslie, D. C., Matthews, B. D., Fraser, J. P., Jurek, S., Hamilton, G. A., ... & Ingber, D. E. (2012). A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Science translational medicine, 4(159), 159ra147-159ra147.
[24] Ma H., Xu H. & Qin J. Biomimetic tumor microenvironment on a microfluidic platform. Biomicrofluidics. 7, 11501 (2013).
[25] Liu T., Lin B., and Qin J., Lab Chip 10, 1671 (2010).
[26] Toley, B. J., Ganz, D. E., Walsh, C. L. & Forbes, N. S. Microfluidic device for recreating a tumor microenvironment in vitro. J. Vis. Exp. 5–9 (2011).
