CH391L/S12/In vitro Selection: Difference between revisions
Adam Meyer (talk | contribs) No edit summary |
Adam Meyer (talk | contribs) No edit summary |
||
| Line 12: | Line 12: | ||
The easiest function to enrich for is affinity for a ligand. To select for binders (aptamers for nucleic acids; antibodies and others for proteins,) one exposes the pool of potential binders to a fixed ligand. The best binders affix to the ligand while weaker binders are washed away. Those that remain can be amplified for further round of selection. | The easiest function to enrich for is affinity for a ligand. To select for binders (aptamers for nucleic acids; antibodies and others for proteins,) one exposes the pool of potential binders to a fixed ligand. The best binders affix to the ligand while weaker binders are washed away. Those that remain can be amplified for further round of selection. | ||
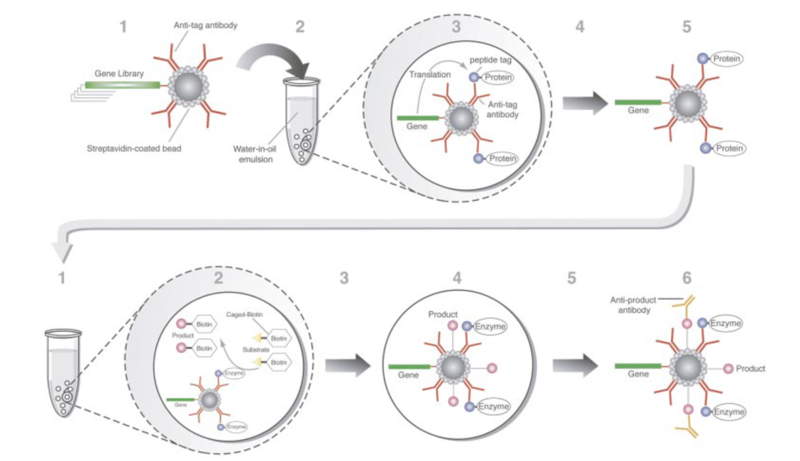
For nucleic acids, the binder itself can be subject to amplification, as it is both the information-carrying and function-carrying molecule. Ellington 1990. | For nucleic acids, the binder itself can be subject to amplification, as it is both the information-carrying and function-carrying molecule. In Ellington and Szostak (1990), A DNA library was created such a T7 promoter drives expression of an N100 RNA pool, flanked by constant regions. The pool is passed through a column with a bound ligand. Species that bind the ligand are retained on the column and weaker binders are washed away. Binders are eluted from the column and amplified to begin a new round. | ||
For protein binders, the scheme must include linking of the information-carrying nucleic acid to the function-carrying protein. Some examples of this linking are phage display, cell-surface display, and ribosome display. | For protein binders, the scheme must include linking of the information-carrying nucleic acid to the function-carrying protein. Some examples of this linking are phage display, cell-surface display, and ribosome display. | ||
| Line 18: | Line 18: | ||
Bartel 1993 | Bartel 1993 | ||
=====Protection===== | =====Protection===== | ||
The first ''in vitro'' evolved protein functions involved modification of the nucleic acid species that encoded it | The first ''in vitro'' evolved protein functions involved modification of the nucleic acid species that encoded it. Perhaps the first such function was protection of the DNA template. In Tawfik and Griffiths (1998) The template encodes HaeIII methyl transferase. Upon transcription and translation in bacterial lysate, active methyltransferase methylate HaeIII recognition sequences in the gene. The methylated genes are then protected from digestion by HaeIII endonuclease. Undigested templates are then amplified before the next round. The key to this experiment is the use of ''in vitro'' compartmentalization (described below)which allow an active methyltransferase to methylate its parent template, but not other templates in the pool. | ||
=====FACS===== | =====FACS===== | ||
Griffiths 2003 [[image:CH391L_S12_Griffiths_2003_schema.png | Griffiths 2003 Schema| 800px]] | Griffiths 2003 [[image:CH391L_S12_Griffiths_2003_schema.png | Griffiths 2003 Schema| 800px]] | ||
===Confinement of Function=== | ===Confinement of Function=== | ||
=====Binding===== | =====Binding===== | ||
=====Cis-action===== | =====Cis-action===== | ||
Revision as of 20:26, 28 January 2012
Overview of in vitro selection
Library Generation
Randomized Oligodeoxynucleotides
Mutagenic PCR
Gene Shuffling
Neutral Drift
Increased Representation
Once diversity is created, the selection must must allow function variants to become a larger percentage of the pool. This step is often the most difficult to design.
Affinity
The easiest function to enrich for is affinity for a ligand. To select for binders (aptamers for nucleic acids; antibodies and others for proteins,) one exposes the pool of potential binders to a fixed ligand. The best binders affix to the ligand while weaker binders are washed away. Those that remain can be amplified for further round of selection.
For nucleic acids, the binder itself can be subject to amplification, as it is both the information-carrying and function-carrying molecule. In Ellington and Szostak (1990), A DNA library was created such a T7 promoter drives expression of an N100 RNA pool, flanked by constant regions. The pool is passed through a column with a bound ligand. Species that bind the ligand are retained on the column and weaker binders are washed away. Binders are eluted from the column and amplified to begin a new round.
For protein binders, the scheme must include linking of the information-carrying nucleic acid to the function-carrying protein. Some examples of this linking are phage display, cell-surface display, and ribosome display.
Selective Amplification
Bartel 1993
Protection
The first in vitro evolved protein functions involved modification of the nucleic acid species that encoded it. Perhaps the first such function was protection of the DNA template. In Tawfik and Griffiths (1998) The template encodes HaeIII methyl transferase. Upon transcription and translation in bacterial lysate, active methyltransferase methylate HaeIII recognition sequences in the gene. The methylated genes are then protected from digestion by HaeIII endonuclease. Undigested templates are then amplified before the next round. The key to this experiment is the use of in vitro compartmentalization (described below)which allow an active methyltransferase to methylate its parent template, but not other templates in the pool.